Other grades of this product :
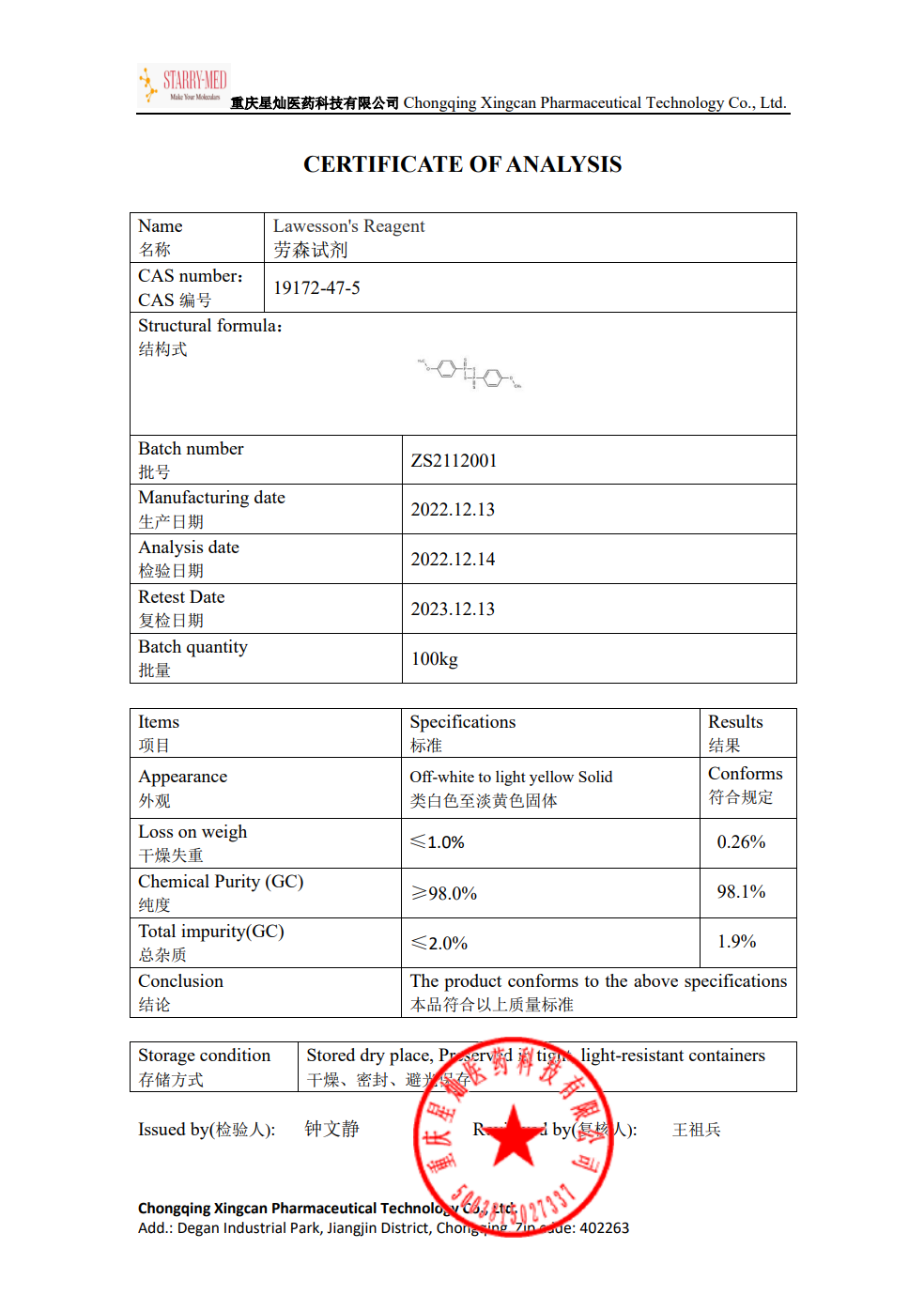
| Melting point | 228-230 °C(lit.) |
| Boiling point | 525.8±60.0 °C(Predicted) |
| density | 1.48±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| form | Powder |
| color | White to almost white |
| Water Solubility | Decomposition |
| Sensitive | Moisture Sensitive |
| Merck | 14,5391 |
| BRN | 1024888 |
| CAS DataBase Reference | 19172-47-5(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3,2,4-Dithiadiphosphetane, 2,4-bis(4-methoxyphenyl)-, 2,4-disulfide (19172-47-5) |
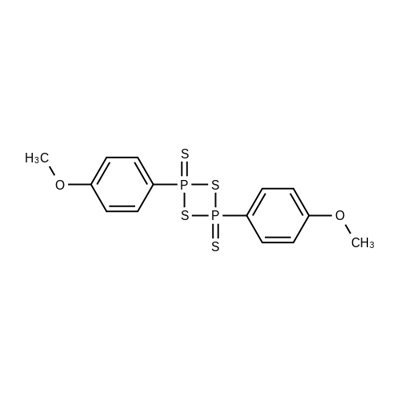
Lawesson's reagent, also known as Lloyd's reagent, is a commonly used chemical reagent in the preparation of organic sulfur compounds. At room temperature under normal pressure, it appears as the solid yellow powder with a strong smell of rotten. In 1956, it was first successfully produced by the reaction between arene and tetraphosphorus decasulfide. The Swedish chemist Sven-Olov Lawesson has carefully studied its reaction with organic compounds, so that its application has been greatly promoted, so the name also derived. The molecule of the Lawesson's reagent contains the four-membered ring structure alternately composed of sulfur and phosphorus.
Upon being heated, it undergoes depolymerization, generating two molecules of unstable ylide (R-PS2), which are the major reactive intermediates. Upon application of the Lawesson’s reagents with two different substitution groups for reaction, it has been observed of molecules with substituent group exchanged with each other in the 31 NMR spectrum of the product, confirming the existence of the intermediates, R-PS2.
Lawesson's reagent is an oxygen-sulfur exchange reagent with the most common application being for the preparation of thioamides and converting carbonyl compounds into sulfur carbonyl compounds. The reacted substrates can include ketone, ester, lactone, amide, lactam, and quinone. The electron-rich carbonyl groups are easier to react. Upon reaction with α, β-unsaturated aldehydes and ketones, the double bond is not affected.