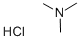Other grades of this product :
| Trimethylamine hydrochloride Basic information |
| Trimethylamine hydrochloride Chemical Properties |
| Melting point | 283-284 °C (dec.)(lit.) | | Boiling point | 132.32°C (rough estimate) | | density | 0.9337 (rough estimate) | | vapor pressure | 0Pa at 25℃ | | refractive index | 1.4413 (estimate) | | storage temp. | Store below +30°C. | | solubility | SOLUBLE | | form | Crystalline Powder | | color | White to slightly cream | | PH | 5 (100g/l, H2O, 20℃) | | Water Solubility | SOLUBLE | | Sensitive | Hygroscopic | | Merck | 14,9710 | | BRN | 3905063 | | InChIKey | SZYJELPVAFJOGJ-UHFFFAOYSA-N | | LogP | -2.25 at 25℃ | | CAS DataBase Reference | 593-81-7(CAS DataBase Reference) | | NIST Chemistry Reference | Methanamine,n,n-dimethyl-,hydrochloride(593-81-7) | | EPA Substance Registry System | Trimethylamine hydrochloride (593-81-7) |
| Trimethylamine hydrochloride Usage And Synthesis |
| Chemical Properties | white to slightly cream crystalline powder | | Uses | In the manufacture of quaternary ammonium Compounds; as insect attractant; as warning agent for natural gas. | | Uses | Trimethylamine hydrochloride is used in the manufacture of quaternary ammonium compounds, as insect attractant. It is used as a raw material for the synthesis of choline chloride, quaternary ammonium compounds, ion exchange resins. It acts as cationic starch reagents, phase transfer catalyst, oil field chemicals, warning agent for natural gas and flotation agent. | | Uses | Trimethylamine hydrochloride is a precursor to prepare aluminium chloride- trimethylamine hydrochloride ionic liquid, which is commonly used in electrodeposition of aluminum wires. It is also used in the synthesis of trimethylamine gallane, trimethylamine-borane and azido-bridged perovskite-type metal-organic frameworks (MOFs). | | Definition | ChEBI: A hydrochloride salt formed by reaction of equimolar amounts of trimethylamine and hydrogen chloride. | | Flammability and Explosibility | Nonflammable | | Purification Methods | The salt crystallises from CHCl3, EtOH or n-propanol, and is dried under vacuum. It also crystallises from *benzene/MeOH, MeOH/diethyl ether and is dried under vacuum over paraffin wax and H2SO4. It is kept over P2O5 as it is hygroscopic.[Beilstein 4 H 262, 4 I 419, 4 IV 138.] |
| Trimethylamine hydrochloride Preparation Products And Raw materials |
|