Other grades of this product :
| P-TOLYL ACETATE Basic information |
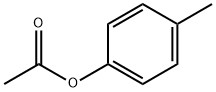
| Product Name: | P-TOLYL ACETATE | | Synonyms: | P-ACETOXYTOLUENE;P-CRESYL ACETATE;P-TOLYL ACETATE;4-Acetoxytoluene;Narceol;Paracresyl acetate;paracresylacetate;p-Cresol acetate | | CAS: | 140-39-6 | | MF: | C9H10O2 | | MW: | 150.17 | | EINECS: | 205-413-1 | | Product Categories: | Organics | | Mol File: | 140-39-6.mol |
| P-TOLYL ACETATE Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36/37/39 | | RIDADR | NA 1993 / PGIII | | WGK Germany | 2 | | RTECS | AJ7570000 | | toxicity | The acute oral LD50 in rats was reported as 1.9 (1.12-3.23) g/kg (Denine, 1973). The acute dermal LD50 in rabbits was reported as 2.1 (1.24-3.57) g/kg (Denine, 1973). |
| P-TOLYL ACETATE Usage And Synthesis |
| Chemical Properties | Colorless, oily liquid; odor similar to
phenol. distillation with
steam. Insoluble in water; soluble in common
organic solvents. Combustible. | | Chemical Properties | p-Tolyl acetate has a strong, floral odor (narcissus) with a characteristic honey-like flavor | | Occurrence | Reported as a constituent of the essential oils of wallflower, cananga and ylang-ylang. | | Uses | Perfumery, flavoring. | | Uses | p-Tolyl acetate has been used in the total synthesis of (?)-incrustoporin. | | Preparation | By acetylation of p-cresol. | | Aroma threshold values | Detection: 25 ppb | | Synthesis Reference(s) | The Journal of Organic Chemistry, 43, p. 2417, 1978 DOI: 10.1021/jo00406a025 | | General Description | The Fries rearrangement of p-tolyl acetate has been investigated using the H-form of various zeolites as catalysts. Mechanism of photo-Fries rearrangement of p-tolyl acetate has been studied. | | Flammability and Explosibility | Notclassified | | Safety Profile | Moderately toxic by
ingestion and skin contact. Combustible
liquid. When heated to decomposition it
emits toxic smoke and irritating fumes. |
| P-TOLYL ACETATE Preparation Products And Raw materials |
|