Other grades of this product :
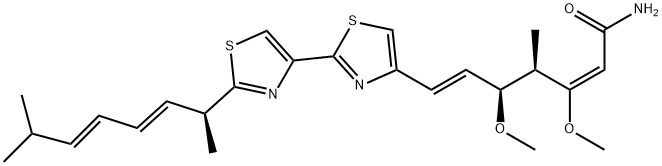
| MYXOTHIAZOL Basic information |
| MYXOTHIAZOL Chemical Properties |
| Boiling point | 679.6±65.0 °C(Predicted) | | density | 1.158±0.06 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | chloroform: soluble9.80 - 10.20mg/mL, clear, colorless to yellow | | pka | 14.07±0.50(Predicted) |
| Hazard Codes | T+ | | Risk Statements | 28 | | Safety Statements | 28-36/37-45 | | RIDADR | UN 3462 6.1/PG 1 | | WGK Germany | 3 | | RTECS | QH7580000 | | F | 10 | | HazardClass | 6.1(b) | | PackingGroup | III |
| MYXOTHIAZOL Usage And Synthesis |
| Uses | Myxothiazol has been used in as a mitochondrial electron transport chain (mETC) inhibitor in P19 murine embryonal carcinoma pluripotent cell line and to treat HeLa cells for integrated stress response activation. | | Definition | ChEBI: A 2,4'-bi-1,3-thiazole substituted at the 4-position with a (1E,3S,4R,5E)-7-amino-3,5-dimethoxy-4-methyl-7-oxohepta-1,5-dien-1-yl] group and at the 2'-position with a (2S | | Biochem/physiol Actions | Myxothiazol, an antibiotic with activity against fungi and insects, is a strong inhibitor of mitochondrial cytochrome b/c1-segment of respiratory chain. Myxothiazol binds to the quinol oxidation (Qo) site of the bc1 complex, blocking electron transfer to the Rieske iron-sulfur protein in the mitochondrial respiratory chain. Oxygen consumption blockage leads to a cytostatic effect that could be reversed. Myxothiazol, as other Epothilones, which are known for their anti tumor activity, contains a thiazole ring that is formed by the incorporation of cysteine into the polyketide backbone. |
| MYXOTHIAZOL Preparation Products And Raw materials |
|