Other grades of this product :
| AMASTATIN HYDROCHLORIDE Basic information |
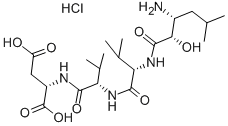
| Product Name: | AMASTATIN HYDROCHLORIDE | | Synonyms: | [(2S,3R)-3-AMINO-2-HYDROXY-5-METHYLHEXANOYL]-VAL-VAL-ASP HYDROCHLORIDE;(2S,3R)-[3-AMINO-2-HYDROXY-5-METHYLHEXANOYL]-VAL-VAL-ASP-OH HCL;AMASTATIN;AMASTATIN HCL;AMASTATIN HYDROCHLORIDE;AMASTATIN HYDROCHLORIDE HYDRATE;H-(2S,3R)-AHMH-VAL-VAL-ASP-OH HCL;N((2S 3R)3-AMINO-2-HXY-5-ME.HEXANOYL)-L& | | CAS: | 100938-10-1 | | MF: | C21H39ClN4O8 | | MW: | 511.01 | | EINECS: | 999-999-2 | | Product Categories: | peptides;inhibitor;Pepetides | | Mol File: | 100938-10-1.mol |
| AMASTATIN HYDROCHLORIDE Chemical Properties |
| Melting point | 202-205 °C | | storage temp. | -20°C | | solubility | methanol: 5 mg/mL, clear, colorless | | form | White to off-white powder. | | BRN | 8888370 |
| WGK Germany | 3 | | F | 10 | | HS Code | 2924190090 |
| AMASTATIN HYDROCHLORIDE Usage And Synthesis |
| Uses | Amastatin is a slow, tight binding, competitive aminopeptidase (AP) inhibitor, first described as an inhibitor of human serum AP-A (glutamyl AP; IC50 = 0.54 μg/ml) but not of AP-B (arginine AP). It also inhibits AP-N (AP-M, alanyl AP; Ki = 20-200 nM), leucyl-cystinyl AP (Ki = 20-220 nM), and endoplasmic reticulum AP 1 (Ki = 41.8 μM). Amastatin is without effect on trypsin, papain, chymotrypsin, elastase, pepsin, or thermolysin.[Cayman Chemical] | | General Description | Chemical structure: peptide | | Biochem/physiol Actions | It inhibits cytosolic leucine aminopeptidase (EC.3.4.11.1), microsomal aminopeptidase M (EC.3.4.11.2) and bacterial leucine aminopeptidase (EC.3.4.11.10). It is less effective against aminopeptidase A (EC 3.4.11.7), the enzyme that converts angiotensin II to angiotensin III. Potentiates the CNS effects of vasopressin and oxytocin in vivo.Effective concentration: 1-10 μM. |
| AMASTATIN HYDROCHLORIDE Preparation Products And Raw materials |
|