Other grades of this product :
| TRIAZAMATE Basic information |
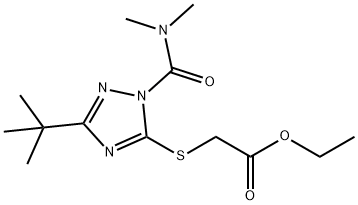
| Product Name: | TRIAZAMATE | | Synonyms: | TRIAZAMATE;APHISTAR;Ethyl (1-dimethylaminocarbonyl-3-(1,1-dimethylethyl)-1H-1,2,4-triazol-5-ylthio)acetate;Ethyl (3-tert-butyl-1-dimethylcarbamoyl-1H-1,2,4-triazol-5-ylthio)acetate;triazamate (bsi,pa e-iso);Acetic acid, ((1-((dimethylamino)carbonyl)-3-(1,1-dimethylethyl)-1H-1,2,4-triazol-5-yl)thio)-, ethyl ester;Acetic acid, ((1-[(dimethylamino)carbonyl]-3-(1,1-dimethylethyl)-1H-1,2,4-triazol-5-yl)thio)-, ethyl ester;Triazamate [iso:bsi] | | CAS: | 112143-82-5 | | MF: | C13H22N4O3S | | MW: | 314.4 | | EINECS: | | Product Categories: | INSECTICIDE | | Mol File: | 112143-82-5.mol |
| TRIAZAMATE Chemical Properties |
| Melting point | 60° | | Boiling point | 280°C (rough estimate) | | density | 1.2357 (rough estimate) | | vapor pressure | 1.6 x l0-4 Pa (25 °C) | | refractive index | 1.6200 (estimate) | | Fp | 189℃ | | pka | 0.48±0.50(Predicted) | | Water Solubility | 433 mg l-1(25 °C) | | form | neat | | BRN | 8422595 | | EPA Substance Registry System | Triazamate (112143-82-5) |
| Hazard Codes | T,N | | Risk Statements | 23-25-36-50 | | Safety Statements | 26-45-61 | | RIDADR | UN2811 - class 6.1 - PG 2 - EHS - Toxic solids, organic, n.o.s., HI: all | | WGK Germany | 3 | | Toxicity | LD50 (14 d) in mice, rats (mg/kg): 61, 50-200 orally; in rats (mg/kg): >5000 dermally (Murray); LC50 (48 h) in daphnia: 0.048 mg/l; (96 h) in bluegill, trout: 1.0, 0.43 mg/l (Murray) |
| TRIAZAMATE Usage And Synthesis |
| Uses | Pesticide. | | Uses | Triazamate is used for the control of aphids by foliar application
on a wide variety of crops. It is suitable for inclusion in integrated pest
management because it is minimally toxic to beneficial insects. | | Uses | Triazamate is used in controlling unpleasant pests.Used in preparation of Thiazole Manganese Zinc compounds and compounds thereof. | | Definition | ChEBI: A triazole insecticide that is 1H-1,2,4-triazole which is substituted at positions 1, 3, and 5 by N,N-dimethylaminocarbonyl, tert-butyl, and (2-ethoxy-2-oxoethyl)sulfanediyl groups, resp
ctively. | | General Description | Triazamate is a carbamoyl triazole compound, widely used as an insecticide and its mode of action involves the inhibition of cholinesterase in insect pests. | | Metabolic pathway | There is only a limited amount of information on the fate of triazamate
currently in the public domain. Metabolism in sugar beet and apple has
been reported. Animal metabolism studies have been conducted but
details are not available. Triazamate is very rapidly metabolised by
hydrolysis, decarbamoylation and further metabolism in all biological
systems studied (PM). | | Degradation | Triazamate is stable under normal conditions and in solution at pH 7
and below. Its DT50 values in buffers are, at pH 5, 7 and 9, 220 days,
49 hours and 1 hour, respectively (PM). Base-catalysed hydrolysis should
initially afford triazamate acid (2) by carboxyl ester cleavage (see
Scheme 1).
The DTM of triazamate on aqueous photolysis at pH 7 is 301 days
(PM) |
| TRIAZAMATE Preparation Products And Raw materials |
|