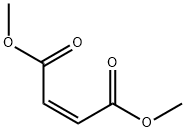
Other grades of this product :
| Dimethyl maleate Basic information |
| Dimethyl maleate Chemical Properties |
| Dimethyl maleate Usage And Synthesis |
| Chemical Properties | colourless liquid | | Uses | Dimethyl maleate is widely used in the plastics and chemical
industries and as a vaporization retardant of insecticides. | | Uses | Dimethyl maleate is acts as a dienophile involved in ultrasonic irradiation promoted Diels-Alder reaction with substituted furans. It is widely used as an intermediate and additive for pharmaceuticals, copolymers, pigments, plastics, paints, adhesives and agrochemicals. As an internal modifier, it increases the glass transition temperature of styrene or vinyl chloride polymer. It plays a vital role to improve the hardness and toughness of polymer films such as copolymers of vinyl acetate. | | Definition | ChEBI: Dimethyl maleate is a maleate ester resulting from the formal condensation of both carboxy groups of maleic acid with methanol. It is commonly used as a dienophile for Diels-Alder-type cycloaddition reactions in organic synthesis. It is a maleate ester, a diester and a methyl ester. It derives from a methanol. | | Preparation | Dimethyl maleate can be synthesized from maleic anhydride and methanol, with sulfuric acid acting as acid catalyst, via a nucleophilic acyl substitution for the monomethyl ester, followed by a Fischer esterification reaction for the dimethyl ester. | | General Description | Dimethyl maleate (DMM) is a reactive dienophile and undergoes ultrasonic irradiation promoted Diels-Alder reaction with substituted furans. Mesoporous siliceous SBA-15-supported Cu catalyzed gas phase hydrogenolysis of DMM to 1,4-butanediol (BDO) has been reported. Aluminium chloride has been reported to accelerate the Diels-Alder reaction of DMM and anthracene. DMM can be synthesized by the esterification of maleic anhydride with sulfuric acid and methanol. | | Flammability and Explosibility | Nonflammable |
| Dimethyl maleate Preparation Products And Raw materials |
|