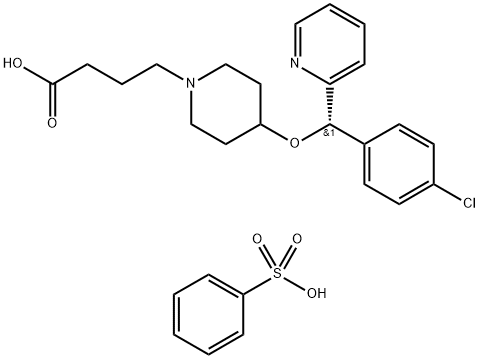
| BEPOTASTINE BESILATE Basic information |
| Product Name: | BEPOTASTINE BESILATE |
| Synonyms: | BEPOTASTINE BESILATE;(+)-(S)-4-(4-((4-Chlorophenyl)(2-pyridyl)methoxy)piperidino)butyric acid monobenzenesulfonate;4-((4-Chlorophenyl)-2-pyridinylmethoxy)- (S)-1-piperidinebutanoic acid monobenzenesulfonate;Talion (TN);Bepotastine Beslilat;Bepotastine Besylate;1-Piperidinebutanoic acid, 4-[(4-chlorophenyl)-2-pyridinylmethoxy]-, (S)-, monobenzenesulfonate;1-Piperidinebutanoic acid, 4-[(S)-(4-chlorophenyl)-2-pyridinylmethoxy]-, monobenzenesulfonate |
| CAS: | 190786-44-8 |
| MF: | C27H31ClN2O6S |
| MW: | 547.06 |
| EINECS: | 1806241-263-5 |
| Product Categories: | Inhibitors;Other APIs;Aromatics;Drug Analogues;Heterocycles;Intermediates & Fine Chemicals;API;Amino Acids & Derivatives;Pharmaceuticals |
| Mol File: | 190786-44-8.mol |
| BEPOTASTINE BESILATE Chemical Properties |
| Melting point | 161-163° |
| alpha | D20 +6.0° (c = 5 in methanol) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| color | White to Light Beige |
| Stability: | Hygroscopic |
| Safety Information |
| MSDS Information |
| BEPOTASTINE BESILATE Usage And Synthesis |
| Description | Betotastine was introduced in Japan for the treatment of allergic rhinitis. This structurally-related derivative of chlorpheniramine and ebastine is prepared by condensation of optically-resolved 4-[1-(4-chlorophenyl)-1-(2-pyridyl)-methoxy]piperidine with ethyl 4-bromobutyrate followed by ester hydrolysis. Betotastine is the seventh marketed non-sedating histamine H1 antagonist. Its very low sedative side effect is due to very poor penetration in the central nervous system. Besides its potent and long-acting activity in models of allergic rhinitis, betotastine was also shown to act as a PAF antagonist and inhibit LTD4 in tracheal smooth muscle and ileum, IL-5 production by human peripheral blood mononuclear cells as well as eosinophil infiltration in the airway and peripheral blood. As a consequence, it is currently being developed against other allergic and respiratory disorders. |
| Description | Bepotastine is an antagonist of the histamine H1 receptor that is selective over H3, α1-, α2-, and β-adrenergic, dopamine D2long, serotonin 5-HT2, muscarinic acetylcholine, and benzodiazepine receptors. It reduces dye leakage from the nasal passages of rats acutely sensitized to an antigen (ED50 = 0.03 mg/kg) and inhibits histamine-induced bronchoconstriction in the anesthetized dog (ED50 = 3.2 μg/kg). Bepotastine prevents conjunctival vascular hyperpermeability in a guinea pig model of conjunctivitis in a dose-dependent manner. Formulations containing bepotastine have been used in the treatment of itching associated with allergic conjunctivitis. |
| Chemical Properties | Off-White to Light Beige Solid |
| Originator | UBE (Japan) |
| Uses | Bepotastine is a non-sedating, selective antagonist of histamine 1 (H1) receptor with pIC50 of 5.7 |
| Uses | Bepotastine is a histamine H1 receptor anatagonist. Bepotastine suppresses some allergic inflammatory processes such as allergic rhinitis, chronic urticaria or pruritus associated with skin conditions (eczema/dermatitis, prurigo or pruritus cutaneus). |
| Definition | ChEBI: An organosulfonate salt obtained by combining equimolar amounts of bepotastine and benzenesulfonic acid. A topical, selective and non-sedating histamine (H1) receptor antagonist used for treatment of itching associated with allergic co junctivitis. |
| Manufacturing Process | Manufacturing process for BEPOTASTINE BESILATE includes these steps as follows: Step A: Synthesis of Methyl 2-endo-hydroxy-1-exo-hydroxymethyl-3a,8b-cis-2,3,3a,8b-tetrahydro- 1H-5-cyclopenta[b]benzofurancarboxylate,Step B: Synthesis of Methyl 3-methyl-trans-4a-cisoid-4a,5a-cis-5a-1,4a,5,5a,10b,10c-hexahydro-7- dioxin o[5,4-a]cyclopenta[b]benzofurancarboxylate,Step C: Synthesis of 3-Methyl-trans-4a-cosoid-4a,5a-cis-5a-1,4a,5,5a,10b,10c-hexahydro-7- dioxino[5,4-a]cyclopenta[b]benzofuranylmethanol,Step D: Synthesis of 7-Chloromethyl-3-methyl-trans-4a-cisoid-4a,5a-cis-5a-1,4a,5,5a,10b,10chexahydrodioxino[5,4-a]cyclopenta[b]benzofuran,Step E: Synthesis of 4-[3-Methyl-trans-4a-cisoid-4a,5a-cis-5a-1,4a,5,5a,10b,10c-hexahydro-7- dioxino[5,4-a]cyclopenta[b]benzofuranyl]butyric acid, Step F: Synthesis of Methyl 4-[2-endo-hydroxy-1-exo-hydroxymethyl-3a,8b-cis-2,3,3a,8btetrahydro-1H-5- cyclopenta[b]benzofuranyl]butyrate, Step G: Synthesis of Methyl 4-[2-endo-acetoxy-1-exo-hydroxymethyl-3a,8b-cis-2,3,3a,8btetrahydro-1H-5-cyclopenta[b]benzofuranyl]butyrate, Step H: Synthesis of Methyl ester of 11,15-dideoxy-11-acetoxy-16-methyl-15-oxo-18,19- tetradehydro-5,6,7-trinor-4,8-inter-m-phenylene PGI2,Step I: Synthesis of 11-Deoxy-11-acetoxy-16-methyl-18,19-tetradehydro-5,6,7-trinor-4,8-inter-mphenylene PGI2.To a solution of 54 mg of methyl ester of 11-deoxy-11-acetoxy-16-methyl- 18,19-tetradehydro-5,6,7-trinor-4,8-inter-m-phenylene PGI2 in 4.5 ml of anhydrous methanol was added 0.001 ml of 4.8 N sodium methoxide under argon, and the reaction mixture was stirred for 1.5 hours at room temperature. After addition of acetic acid to the reaction mixture and concentration of the mixture, the residue was dissolved in 20 ml of ethyl acetate, and the solution was washed with aqueous saturated solution of sodium hydrogen carbonate, water and aqueous saturated solution of sodium chloride, dried and concentrated to afford 55 mg of an oily material. This oily material was purified by column chromatography using ethyl acetate and cyclohexane (3:1) as eluent to give 48 mg of the methyl ester of 16- methyl-18,19-tetradehydro-5,6,7-trinor-4,8-inter-m-phenylene PGI2. |
| Brand name | Talion |
| Therapeutic Function | Antiallergic |
| BEPOTASTINE BESILATE Preparation Products And Raw materials |
Please leave a message for us or use the following ways to contact us, we will reply to you as soon as possible, and provide you with the most sincere service, thank you.