| Vorapaxar Sulfate Basic information |
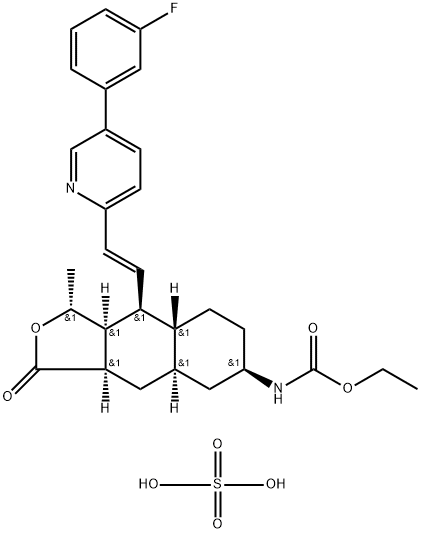
| Product Name: | Vorapaxar Sulfate |
| Synonyms: | Vorapaxar Sulfate;N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-Fluorophenyl)-2-pyridinyl]ethenyl]dodecahydro-1-Methyl-3-oxonaphtho[2,3-c]furan-6-yl]carbaMic Acid Ethyl Ester Sulfate;Sch 530348;SCH 530348 (H2SO4 Salt);vorapaxar impurity A;Carbamic acid, N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)-2-pyridinyl]ethenyl]dodecahydro-1-methyl-3-oxonaphtho[2,3-c]furan-6-yl]-, ethyl ester, sulfate (1:1);SCH-530348;SCH530348;Vorapaxar Sulfate (SCH 530348) |
| CAS: | 705260-08-8 |
| MF: | C29H35FN2O8S |
| MW: | 590.66 |
| EINECS: | |
| Product Categories: | Aromatics;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals |
| Mol File: | 705260-08-8.mol |
| Vorapaxar Sulfate Chemical Properties |
| Safety Information |
| MSDS Information |
| Vorapaxar Sulfate Usage And Synthesis |
| Description | Merck Sharp & Dohme successfully obtained approval in the EU in 2014 for vorapaxar sulfate, marketed as Zontivity®. The drug is a first-in-class thrombin receptor (also referred to as a protease-activated or PAR-1) antagonist which, when used in conjunction with antiplatelet therapy, has been shown to reduce the chance of myocardial infarction and stroke, particularly in patients with a history of cardiac events. Antagonism of PAR-1 allows for thrombin-mediated fibrin deposition while blocking thrombinmediated platelet activation. |
| Uses | SCH-530348 is a novel antiplatelet agent undergoing development by Schering-Plough Corp for the treatment and prevention of atherothrombosis. acute coronary syndrome (unstable angina/non-ST segment elevation myocardial infarction) and secondary prevention of cardiovascular events in high-risk patients. |
| Definition | ChEBI: An organic sulfate salt obtained by combining vorapaxar with one molar equivalent of sulfuric acid. A protease-activated receptor-1 antagonist used for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (M ) or with peripheral arterial disease. It has been shown to reduce the rate of a combined endpoint of cardiovascular death, MI, stroke and urgent coronary revascularisation. |
| Synthesis | Although a variety of papers and patents describe the synthesis of vorapaxar sulfate (XXXVII), a combination of two patents describe the largest-scale synthesis reported in the literature. Retrosynthetically, the drug can be divided into olefination partners 306 and 305. Lactone 305 is further derived from synthons 300 and 299, which are readily prepared from commercially available starting materials. Dienyl acid 300 was constructed in two steps starting from commercial vinyl bromide 307, which first undergoes a Heck reaction with methacrylate (308) followed by saponification of the ester to afford the desired acid 300 in 71% over two steps. The synthesis of alcohol 299 begins with tetrahydropyranyl (THP) protection of enantioenriched alcohol 295 to afford butyne 297 . Lithiation of this system followed by trapping with (benzyloxy)chloroformate and Dowex work-up to remove the protective functionality provided acetyl ester 298. Hydrogenation of the alkyne with Lindlar’s catalyst delivered cis-allylic alcohol 299 in 93% yield. Acid 300 was then esterified with alcohol 299 by way of a 1,3-dicyclohexylcarbodiimide (DCC) coupling and, upon heating in refluxing xylenes, an intramolecular Diels– Alder reaction occurred. Subsequent subjection to DBU secured the tricyclic system 301 in 38% over three steps as a single enantiomer. Diastereoselective hydrogenation reduced the olefin with concomitant benzyl removal to give key fragment 302. Next, acidic revelation of the ketone followed by reductive amination with ammonium formate delivered primary amines 303a/303b as a mixture of diastereomers. These amines were then converted to the corresponding carbamates, and resolution by means of recrystallization yielded 50% of 304 as the desired diastereomer. Acid 304 was treated with oxalyl chloride and the resulting acid chloride was reduced to aldehyde 305 in 66% overall yield. Finally, deprotonation of phosphonate ester 306 followed by careful addition of 305 and acidic quench delivered vorapaxar sulfate (XXXVII) in excellent yield over the two-step protocol. The preparation of vorapaxar phosponate ester 306 commenced from commercial sources of 5-(3-fluorophenyl)-2- methylpyridine (310). Removal of the methyl proton with LDA followed by quench with diethyl chlorophosphonate resulted in phosponate ester 306. |
| Vorapaxar Sulfate Preparation Products And Raw materials |
Please leave a message for us or use the following ways to contact us, we will reply to you as soon as possible, and provide you with the most sincere service, thank you.