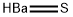Other grades of this product :
| BARIUM SULFIDE Chemical Properties |
| Melting point | 1200°C | | density | 4.25 g/mL at 15 °C(lit.) | | form | Powder | | Specific Gravity | 4.25 | | color | Pale gray to yellow | | Water Solubility | Soluble in water but insoluble in alcohol. | | Sensitive | Moisture Sensitive | | Merck | 14,995 | | Stability: | Stable, but decomposes on heating. Incompatible with acids, oxidizing agents, water. | | CAS DataBase Reference | 21109-95-5(CAS DataBase Reference) | | NIST Chemistry Reference | Barium sulfide(21109-95-5) | | EPA Substance Registry System | Barium sulfide (21109-95-5) |
| BARIUM SULFIDE Usage And Synthesis |
| Description | Barium sulfide (BaS) belongs to the inorganic compound that possesses different characters, which allows it to be applied in a variety of fields. It is primarily used as an important precursor to produce other barium compounds, such as barium carbonate as well as the pigment lithopone (ZnS•BaSO4). Similar to other chalcogenides of the alkaline earth metals, barium sulfide can be employed as a short short wavelength emitter for electronic displays and used in electronics, paint pigments, dehairing hides, flame retardant, luminous paints, and producing pure hydrogen sulfide. Besides, barium sulfide is effective to deviate X-rays and helps to give clearly image of the soft tissue.
| | References | https://en.wikipedia.org/wiki/Barium_sulfide
https://www.alfa.com/zh-cn/catalog/012848/
https://www.merriam-webster.com/dictionary/barium%20sulfide
| | Chemical Properties | colourless crystals, or white to grey-brown powder, | | Physical properties | Colorless crystalline solid; density 4.25 g/cm3; refractive index 2.155; melts at 1,200°C; soluble in water (decomposes); insoluble in alcohol. | | Occurrence | Barium sulfide occurs in the form of black ash, which is a gray to black impure product obtained from high temperature carbonaceous reduction of barite. It is the starting material in the manufacture of most barium compounds including barium chloride and barium carbonate. It is used in luminous paints; for dehairing hides; as a flame retardant; and for generating H2S. | | Uses | As depilatory; in luminous paints; manufacture of lithopone; vulcanizing rubber, generating H2S. | | Uses | Barium sulfide has different characters, so it is used in a variety of ways, such as in electronics, paint pigments, dehairing hides, flame retardant, luminous paints, and generating pure hydrogen sulfide. Barium sulfide can deviate X-rays and helps to give clearly image of the soft tissue. Barium sulfide is a precursor to other barium compounds and also used as a short wavelength emitters for electronic displays. | | Production Methods | Barium sulfide is grayish-white solid, formed by heating barium sulfate and carbon, reactive with H2O to form barium hydrosulfide, Ba(SH)2, solution. The latter is also made by saturation of barium hydroxide solution with H2S. Barium polysulfides are formed by boiling barium hydrosulfide with sulfur. | | Preparation | Barium sulfide can be
prepared by the direct reaction of the elements, calcined
in an inert atmosphere, at a 1:1.05 molecular ratio:
Ba+S+heat→BaS
Barium sulfide is prepared commercially by heating
barite (BaO) with coal or petroleum coke in a rotary kiln
at 1000°C to 1250°C in an oxygen-free atmosphere. The
product, black ash, is a gray or black powder containing
carbonaceous impurities and unreacted barite. Barium
sulfide is separated from impurities by extraction with
hot water and filtration. Barium sulfide may also be made by high temperature reduction of barium sulfate with methane. | | General Description | Barium sulfide (BaS) is an inorganic compound that is used as a precursor in the production of barium compounds. These compounds can be used in a variety of applications, such as ceramics, luminous paints, additives, and flame retardants. | | Hazard | Highly toxic by ingestion (see Barium). | | Flammability and Explosibility | Nonflammable | | Safety Profile | A poison. Flammable
by spontaneous chemical reaction, air,
moisture, or acid fumes may cause it to
ignite. For explosion and disaster hazards,
see SULFIDES. To fight fire, use CO2, dry
chemical. Reacts violently with
phosphorus(V) oxide. Mxtures with lead
dioxide, potassium chlorate, or potassium
nitrite explode when heated. Incompatible
with Cl2O, Ca(NO3)2, r(NO3)2, Ca(ClO3)2,
Sr(ClO3)2, (ClO3)2. See also BARIUM
COMPOUNDS (soluble) and SULFIDES. |
| BARIUM SULFIDE Preparation Products And Raw materials |
|