Other grades of this product :
| (Benzyloxycarbonylmethyl)triphenylphosphonium bromide Basic information |
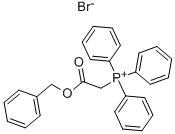
| Product Name: | (Benzyloxycarbonylmethyl)triphenylphosphonium bromide | | Synonyms: | (BENZYLOXYCARBONYLMETHYL)TRIPHENYLPHOSPHONIUM BROMIDE;(Benzyloxycarbonylmethyl)triphenylphosphonium bromide, 98 %;(benzyloxycarbonylmethyl)triphenyl-phosphonium br;triphenyl benzoxycarbonyl Methyl phosphoniuM broMide;(2-(Benzyloxy)-2-oxoethyl)triphenylphosphonium bromide;[2-oxo-2-(benzyloxy)ethyl]triphenyl-phosphonium bromide;triphenyl benzoxycarbonyl Methyl phosphon;(2-oxo-2-phenylmethoxyethyl)-triphenylphosphonium bromide | | CAS: | 78385-36-1 | | MF: | C27H24BrO2P | | MW: | 491.36 | | EINECS: | | Product Categories: | C-C Bond Formation;Olefination;Wittig Reagents | | Mol File: | 78385-36-1.mol |
| (Benzyloxycarbonylmethyl)triphenylphosphonium bromide Chemical Properties |
| Melting point | 124 °C (dec.)(lit.) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 |
| (Benzyloxycarbonylmethyl)triphenylphosphonium bromide Usage And Synthesis |
| Uses | Reactant for:- Atypical aza-Morita-Baylis-Hillman mechanism
- Thermal decomposition
- Double aza-Michael reaction
- Cyclodextrin derivatives
- Stereoselective preparation of α,β-unsaturated carbonyl compounds via stereoselective Wittig olefination with aldehydes under solventless conditions or ultrasonication
- Synthesis of [difluoro(alkenylphenyl)methyl]phosphonic acids on non-crosslinked polystyrene as inhibitors of PTP-1B via Wittig reaction
|
| (Benzyloxycarbonylmethyl)triphenylphosphonium bromide Preparation Products And Raw materials |
|