| Description | Arachidonic acid belongs to a kind of polyunsaturated omega-6 fatty acid, which is highly biologically relevant. It is abundantly distributed in brain, muscles and liver. It is the precursor for all prostaglandins, thromboxanes, and leukotrienes. Most cellular arachidonic acid is esterified in the membrane phospholipids. It is an important second messenger of cellular signalling participating in the regulation of signaling enzymes including PLC-γ, PLC-δ, and PKC-α, -β, and -γ isoforms. In addition, arachidonic acid acts as key inflammatory intermediate as well as avasodilator. Generally, the body can synthesize the arachidonic acid through linoleic acid. However, upon linoleic acid deficiency, it is necessary to supplement arachidonic acid from the diets. Food sources of arachidonic acid include meat, eggs and some fishes. Alternatively, we can also have arachidonic acid supplements. |
| References | http://www.fitday.com/fitness-articles/nutrition/healthy-eating/what-is-arachidonic-acid.html
https://en.wikipedia.org/wiki/Arachidonic_acid
https://www.caymanchem.com/product/90010 |
| Description | Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6). It is the counterpart to the saturated arachidic acid found in peanut oil, (L. arachis – peanut.). |
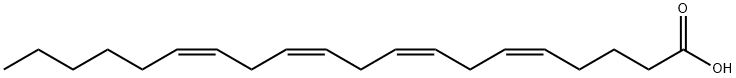
| Chemical Properties | In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four cis-double bonds; the first double bond is located at the sixth carbon from the omega end. Some chemistry sources define 'arachidonic acid' to designate any of the eicosatetraenoic acids. However, almost all writings in biology, medicine and nutrition limit the term to all-cis-5,8,11,14- eicosatetraenoic acid. |
| Chemical Properties | liquid |
| Uses | An unsaturated omega-6 fatty acid constituent of the phospholipids of cell membranes |
| Uses | Arachidonic Acid is an essential fatty acid and a precursor in the biosynthesis of prostaglandins, thromboxanes, and leukotrienes. Arachidonic Acid occurs in liver, brain, glandular organs, and depot
fats of animals, in small amounts in human depot fats, and Arachidonic Acid is also a constituent of animal phosphatides. |
| Uses | arachidonic acid is an ingredient with skin-smoothing, emollient, and healing properties. Arachidonic acid is an omega-6 essential fatty acid naturally occurring in the skin and considered critical for appropriate skin metabolism. It is a constituent of vitamin F. |
| Definition | ChEBI: A long-chain fatty acid that is a C20, polyunsaturated fatty acid having four (Z)-double bonds at positions 5, 8, 11 and 14. |
| General Description | A Certified Spiking Solution? suitable for use in mass spectrometry-based fatty acid testing applications such as assessment of cardiovascular disease risk and fatty acid deficiency, and detection and quantification of arachidonic acid in nutraceuticals and dietary supplements. Arachidonic acid (cis-5,8,11,14), sometimes referred to as AA or ARA, is a polyunsaturated omega-6 fatty acid. Studies suggests that ARA as well as other fatty acids can serve as biomarkers for cardiovascular disease, and nutritional and metabolic disorders. |
| Biological Activity | Endogenous free fatty acid released from phospholipids by phospholipase A 2 . Important cellular signaling mediator and precursor of eicosanoids. Metabolized by lipoxygenases, cyclooxygenases and cytochrome P450 monooxygenases. |
| Biochem/physiol Actions | Arachidonic acid stimulates adhesion of MDA-MB-435 human metastatic cancer cells to extracellular matrix molecules (collagen IV and vitronectin) . |
| Safety Profile | Poison by intravenous route.Experimental reproductive effects. Mutation datareported. When heated to decomposition it emits acridsmoke and irritating fumes. |
| Carcinogenicity | In vitro and in vivo studies indicate
that inhibition of arachidonic acid metabolism inhibits
the growth of malignant cells, including head and neck
squamous cell carcinoma, and implicates arachadonic acid
in facilitating the metastasis of these tumor cells.
Arachidonic acid reaction with cyclooxygenase and lipoxygenases
yield eicosanoids that can mediate prostate cancer
proliferation and enhance both tumor vascularization
and metastasis. Cytochrome P450 arachidonic acid
epoxygenases promote cell proliferation and inhibit apoptosis
in endothelial cells. |