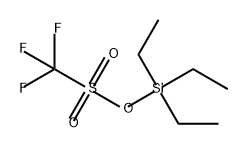
Other grades of this product :
| Triethylsilyl trifluoromethanesulfonate Basic information |
| Triethylsilyl trifluoromethanesulfonate Chemical Properties |
| Boiling point | 85-86 °C/12 mmHg (lit.) | | density | 1.169 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.389(lit.) | | Fp | 162 °F | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Triethylsilyl Trifluoromethanesulfonate is readily sol hydrocarbons, dialkyl ethers, halogenated
solvents. CH2Cl2 is employed most commonly. Reactions in
1,2-dichloroethane proceed faster than those in CCl4 or Et2O.
Protic solvents and THF react with trialkylsilyl triflates and are
therefore not suitable. | | form | Fuming Liquid | | Specific Gravity | 1.169 | | color | Clear colorless to light brown | | Water Solubility | Miscible with dichloromethane, hydrocarbons, dialkyl ethers and halogenated solvents. Immiscible with water. | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 3590541 | | CAS DataBase Reference | 79271-56-0(CAS DataBase Reference) |
| Hazard Codes | C,F | | Risk Statements | 34-14 | | Safety Statements | 26-36/37/39-45-27 | | RIDADR | UN 3265 8/PG 2 | | WGK Germany | 3 | | F | 10-21 | | Hazard Note | Corrosive/Flammable | | TSCA | No | | HazardClass | 8 | | PackingGroup | II | | HS Code | 29319090 |
| Triethylsilyl trifluoromethanesulfonate Usage And Synthesis |
| Chemical Properties | clear colorless to light brown fuming liquid | | Physical properties | 85–86 °C/12 mmHg; d 1.169 g cm?3. | | Uses | Triethylsilyl Trifluoromethanesulfonate can be used as potent silylating agent and as Lewis acid catalyst. Triethylsilyl ethers are generally
more stable towards hydrolysis than are trimethylsilyl ethers, and
consequently the Et3Si moiety has gained increasing use as a protecting group for alcohols. However, since it is often difficult to
silylate sterically hindered hydroxyl groups using Et3SiCl, triethylsilyl perchlorate and triethylsilyl triflate (Et3SiOTf) were introduced to overcome this problem. Jefford has reported that condensation
reactions of 2-trimethylsiloxyfuran with aldehydes can be catalyzed by Et3SiOTf to give mainly the threo addition product
(eq 8). | | Uses | Triethylsilyl trifluoromethanesulfonate acts as a silylating agent. It is also useful as a Lewis acid catalyst. Further, it reacts with 1-diazo-3,3-dimethyl-butan-2-one to prepare 1-diazo-3,3-dimethyl-1-(triethylsilyl)-2-butanone. | | Preparation | Triethylsilyl Trifluoromethanesulfonate can be prepared by reacting chlorotriethylsilane with trifluoromethanesulfonic acid followed by
distillation. |
| Triethylsilyl trifluoromethanesulfonate Preparation Products And Raw materials |
|