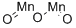Other grades of this product :
| MANGANESE (III) OXIDE Basic information |
| Product Name: | MANGANESE (III) OXIDE | | Synonyms: | MANGANESE (III) OXIDE, 99.9%;Manganese(III)oxide,99%;Manganese(III) oxide, 99.999% metals basis;MANGANESE(III) OXIDE, -325 MESH, 99%;MANGANESE (III) OXIDE, MN>70+% 300 MESH (POWDER 99%);keto(ketomanganiooxy)manganese;oxo(oxomanganiooxy)manganese;Manganese(III) oxide 99.99% trace Metals basis | | CAS: | 1317-34-6 | | MF: | Mn2O3 | | MW: | 157.87 | | EINECS: | 215-264-4 | | Product Categories: | Inorganics;metal oxide | | Mol File: | 1317-34-6.mol |
| MANGANESE (III) OXIDE Chemical Properties |
| Melting point | 1080°C (dec.) | | density | 4.5 g/mL at 25 °C(lit.) | | form | Powder | | color | Black | | Specific Gravity | 4.5 | | Water Solubility | Soluble in acid and ammonium chloride. Insoluble in water, alcohol and acetone. | | Merck | 14,5737 | | Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3OSHA: Ceiling 5 mg/m3NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 | | InChIKey | GEYXPJBPASPPLI-UHFFFAOYSA-N | | CAS DataBase Reference | 1317-34-6(CAS DataBase Reference) | | EPA Substance Registry System | Manganese(III) oxide (1317-34-6) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | OP0915000 | | TSCA | Yes |
| MANGANESE (III) OXIDE Usage And Synthesis |
| Chemical Properties | manganese(III) oxide is a black, lustrous powder, sometimes tinged brown. Very hard.decomposes at 1080℃. Soluble in cold hydrochloric acid; not soluble in nitric acid (decomposes), hot sulfuric acid; insoluble in water. Noncombustible.
manganese(III) oxide is obtained by igniting manganese(IV) oxide or a manganese(II) salt in air at 800°C. It reacts slowly with cold dilute acids to form manganese(III) salts. Manganese(III) oxide occurs in nature as braunite (3Mn2O3.MnSiO3 ) and the monohydrate (Mn2O 3.H2O) occurs in the mineral manganite. The manganese(III) oxide crystal lattice contains Mn3+ and O2– ions. With concentrated alkalis, it undergoes disproportionation to give manganese(II) and manganese(IV) ions.
| | Uses | Manganese(III) oxide is used to produce ceramic magnets and semiconductors. Further, it serves as a precursor to lithium manganese oxide. In addition to this, it acts as a useful battery cathode. | | Preparation | Anhydrous manganese(III) oxide is found as the mineral braunite, Mn2O3, while a hydrated form, Mn2O3.H2O, which is chemically more reactive, occurs as manganite. Manganese forms both anions and cations in oxidation state +3. Simple salts of tervalent manganese are not stable. However, complexes are more stable, the hexacyanomanganates(III) being an example: K3Mn(CN)6 is a red crystalline solid, isomorphous with potassium hexacyanoferrate(III) and slowly hydrolysed.
Manganese(III) oxide is formed as a grey solid by heating manganese(IV) oxide to 700°C for several hours:
4MnO2 ---> 2Mn203 + O2
It is converted into trimanganese tetroxide when heated above 940°C in air or reduced by hydrogen above 230°C. Hot concentrated hydrochloric acid is oxidized to chlorine by manganese(III) oxide:
Mn203 (s) + 6H + + 2e- ==2Mn2+ + 3H2O
Mn203 (s) + 6H + + 2Cl- ---> 2Mn2+ + 3H2O + Cl2 | | Definition | In nature as manganite,
a manganese ore. | | Hazard | Flammable in finely divided form. Toxic by
inhalation of dust. | | Flammability and Explosibility | Notclassified | | Safety Profile | Moderately toxic by
subcutaneous and intratracheal routes. See
also MANGANESE COMPOUNDS. |
| MANGANESE (III) OXIDE Preparation Products And Raw materials |
|