Other grades of this product :
| DBCO-amine Basic information |
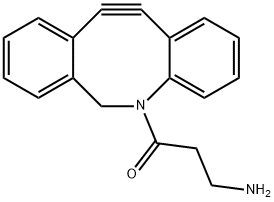
| Product Name: | DBCO-amine | | Synonyms: | Dibenzocyclooctyne-aMine for Copper-free Click CheMistry;ADIBO-CH2CH2NH2·TFA;Azadibenzocyclooctyne-(CH2)2-amine TFA;DBCO-amine;DBCO-NH2;Dibenzocyclooctyne-amine;DBCO-(CH2)2-NH2.TFA;DBCO-(CH2)3-NH2.TFA | | CAS: | 1255942-06-3 | | MF: | C18H16N2O | | MW: | 276.33 | | EINECS: | | Product Categories: | DBCO | | Mol File: | 1255942-06-3.mol |
| DBCO-amine Chemical Properties |
| Melting point | 86-96°C | | Boiling point | 549.9±50.0 °C(Predicted) | | density | 1.25±0.1 g/cm3(Predicted) | | storage temp. | -20°C | | pka | 8.81±0.10(Predicted) | | form | Solid | | color | Yellow to Orange | | InChIKey | PQFQVUMDOWUGMK-CHMOPDDRSA-N |
| WGK Germany | 3 | | HS Code | 29339900 |
| DBCO-amine Usage And Synthesis |
| Description | DBCO-amine is a simple building block containing a DBCO moiety and will add minimal spacer to the modified molecules. In the presence of activators such as EDC or HATU, this reagent can be used to derivatize carboxyl groups or activated esters (e.g. The NHS ester) through a stable amide bond. DBCO is commonly used for copper-free Click Chemistry reactions. | | Uses | Azadibenzocyclooctyne amine is a carbonyl reactive reagent used to incorporate ADIBO into organic compounds, surfaces or particlesAzadibenzocyclooctyne amine is widely useful in strain-promoted copper-free azide-alkyne cycloaddition reactions. It reacts with azide functionalized compounds or bimolecules to give stable triazole linkage without a need for a Cu(I) catalyst. | | Uses | DBCO-Amine was useful for the study of labelled T-cells, which were used to predict the therapeutic efficacy of adoptive T-cell therapy. |
| DBCO-amine Preparation Products And Raw materials |
|