Other grades of this product :
| Isophthalic acid Basic information |
| Isophthalic acid Chemical Properties |
| Melting point | 341-343 °C (lit.) | | Boiling point | 214.32°C (rough estimate) | | density | 1,54 g/cm3 | | vapor pressure | 0Pa at 25℃ | | refractive index | 1.5100 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | 0.12g/l | | form | Crystalline Powder | | pka | 3.54(at 25℃) | | color | White to off-white | | PH | 3.33(1 mM solution);2.76(10 mM solution);2.24(100 mM solution) | | Water Solubility | 0.01 g/100 mL (25 ºC) | | Merck | 14,5197 | | BRN | 1909332 | | Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. | | InChIKey | QQVIHTHCMHWDBS-UHFFFAOYSA-N | | LogP | 1.66 at 25℃ | | CAS DataBase Reference | 121-91-5(CAS DataBase Reference) | | NIST Chemistry Reference | 1,3-Benzenedicarboxylic acid(121-91-5) | | EPA Substance Registry System | Isophthalic acid (121-91-5) |
| Isophthalic acid Usage And Synthesis |
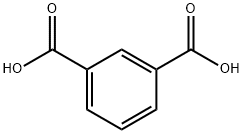
| Description | Isophthalic acid is an organic compound with the formula C6H4(CO2H)2. This colourless solid is an isomer of phthalic acid and terephthalic acid. These aromatic dicarboxylic acids are used as precursors (in form of acyl chlorides) to commercially important polymers, e.g. the fire-resistant material Nomex. Mixed with terephthalic acid, iso phthalic acid is used in the production of resins for drink bottles. The high-performance polymer poly benzimidazole is produced from iso phthalic acid.
| | Chemical Properties | Isophthalic acid is a white crystalline powder or needle-like crystals and it’s an isomer of phthalic acid and terephthalic acid.It is insoluble in cold water but soluble in oxygenated solvents and alcohol. It is combustible and finely dispersed particles will form explosive mixtures in air.
Isophthalic Acid (PIA) is mainly used in the production of bottle PET, also used in the production of alkyd resin, polyester resin, also used in the production of photosensitive materials, pharmaceutical intermediates and so on.
One of the largest applications for PIA is in unsaturated polyester resins for high-quality gel coats. The hardness, stain and detergent resistance characteristics of PIA are ideal for polyester solid-surface countertops that are an inexpensive alternative to acrylics.
| | Uses | Isophthalic acid is used as an intermediate for high performance unsaturated polyesters, resins for coatings, high solids paints, gel coats and modifier of polyethylene terephthalate for bottles. It acts as precursors for the fire-resistant material nomex as well as used in the preparation of high-performance polymer polybenzimidazole. It is also employed as an input for the production of insulation materials. | | Uses | Purified Isophthalic Acid (PIA) is mainly used as intermediate for high performance UPR, resins for coatings, high solids paints, gel coats, modifier of PET for bottles. Product Data Sheet | | Preparation | Iso phthalic acid is produced on the billion kilogram per year scale by oxidizing meta-xylene using oxygen . The process employs a cobalt-manganese catalyst. In the laboratory, chromic acid can be used as the oxidant. It also arises by fusing potassium meta-sulpho benzoate , or meta - brom benzoate with potassium formate (terephthalic acid is also formed in the last case). The barium salt (as its hexa hydrate) is very soluble (a distinction between phthalic and terephthalic acids). Uvitic acid, 5- methylisophthalic acid, is obtained by oxidizing mesitylene or by condensing pyroracemic acid with baryta water. | | Definition | ChEBI: A benzenedicarboxylic acid that is benzene substituted by carboxy groups at position 1 and 3. One of three possible isomers of benzenedicarboxylic acid, the others being phthalic and terephthalic acids. | | Synthesis Reference(s) | Journal of the American Chemical Society, 82, p. 1911, 1960 DOI: 10.1021/ja01493a020 | | General Description | White solid with a slight unpleasant odor. Sinks in water. | | Air & Water Reactions | Dust forms explosive mixture in air [USCG, 1999]. | | Reactivity Profile | Isophthalic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Isophthalic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. | | Health Hazard | May cause slight to moderate irritation of eyes, skin, and mucous membranes on prolonged contact. Ingestion may cause gastrointestinal irritation. | | Fire Hazard | Behavior in Fire: Dust forms explosive mixture in air. | | Flammability and Explosibility | Nonflammable | | Purification Methods | Crystallise the acid from aqueous EtOH. [Beilstein 9 IV 3292.] |
| Isophthalic acid Preparation Products And Raw materials |
| Raw materials | Acetic acid-->Potassium permanganate-->m-Xylene-->Cobalt acetate-->1,3-Dicyanobenzene-->MANGANESE(II) ACETATE TETRAHYDRATE-->Methyl 3-bromobenzoate-->3-Carboxybenzaldehyde | | Preparation Products | 1,3-Dicyanobenzene-->2-Iodoxybenzoic acid-->Sodium dimethyl 5-sulphonatoisophthalate-->2,6-Dicyano-4-Nitroaniline-->Dimethyl isophthalate-->2,4,5-Trifluorobenzoic acid-->5-AMINO-N-METHYLISOPHTHALAMIC ACID-->5-Nitroisophthalic acid-->5-Amino-2,4,6-triiodo-N-methylisophthalamic Acid-->DIETHYL ISOPHTHALATE-->polyester fibre coloring modifier-->DIMETHYL 5-BROMOISOPHTHALATE-->Biphenyl-3,4′,5-tricarboxylic acid-->cis-1,3-cyclohexanedicarboxylic acid |
|