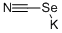Other grades of this product :
| POTASSIUM SELENOCYANATE Basic information |
| POTASSIUM SELENOCYANATE Chemical Properties |
| Melting point | 147 °C | | density | 2.347 | | storage temp. | Inert atmosphere,Room Temperature | | form | Crystalline Solid, Powder, Crystals and/or Chunks | | color | White to off-white | | Specific Gravity | 2.347 | | Water Solubility | Soluble in water, ethanol, dimethyl formamide, hexamethylphosphoramide, acetonitrile and methanol. Slightly soluble in terahydrofuran. | | Sensitive | Moisture Sensitive | | BRN | 3912121 | | Exposure limits | ACGIH: TWA 0.2 mg/m3NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 | | InChIKey | KYEKHFSRAXRJBR-UHFFFAOYSA-M | | CAS DataBase Reference | 3425-46-5(CAS DataBase Reference) | | EPA Substance Registry System | Selenocyanic acid, potassium salt (3425-46-5) |
| POTASSIUM SELENOCYANATE Usage And Synthesis |
| Chemical Properties | white to light beige crystalline powder or needles | | Uses | Potassium selenocyanate is used to prepare triphenylphosphine selenide by reacting with triphenylphosphine. It finds application in solid state NMR of heavy-metal nuclei which results in the preparation of several mercury thiocyanate and selenocyanate compounds. | | Uses | Increasing interest in solid state NMR of heavy-metal nuclei results in the preparation of several Hg-thiocyanate and selenocyanate compounds as potential models. Bowmaker, G.A. et al. Inorg. Chem. 1998, 37, 1734. | | Uses | Increasing interest in solid state NMR of heavy-metal nuclei results in the preparation of several Hg-thiocyanate and selenocyanate compounds as potential models. | | Safety Profile | Poison by
intraperitoneal route. When heated to
decomposition it emits very toxic fumes of
NOx, CN-, K2O, and Se. See also
SELENIUM COMPOUNDS and
CYANATES. | | Purification Methods | Dissolve it in acetone, filter and precipitate it by adding Et2O. |
| POTASSIUM SELENOCYANATE Preparation Products And Raw materials |
|