Other grades of this product :
| 1-Phenyl-1,2-propanedione Basic information |
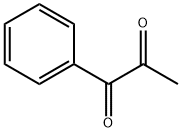
| Product Name: | 1-Phenyl-1,2-propanedione | | Synonyms: | 2-propanedione;Phenyl-1,2-propanedinone;1-PHENYL-1 2-PROPANEDIONE 98+%;Phenyl-1,2-propanedione;1,2-Propanedione, 1-phenyl-;1-Phenyl-1,2-propaned;1-PHENYL-1,2-PROPANEDIONE 98%;Phenyl-1,2-propanedione, 1- | | CAS: | 579-07-7 | | MF: | C9H8O2 | | MW: | 148.16 | | EINECS: | 209-435-2 | | Product Categories: | Organics | | Mol File: | 579-07-7.mol |
| 1-Phenyl-1,2-propanedione Chemical Properties |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36 | | RIDADR | 1224 | | WGK Germany | 3 | | HazardClass | 3 | | PackingGroup | II | | HS Code | 29143990 |
| 1-Phenyl-1,2-propanedione Usage And Synthesis |
| Chemical Properties | 1-Phenyl-1,2-propanedione has a pungent, plastic odor. | | Chemical Properties | clear yellow liquid | | Occurrence | Reported found as a constituent in coffee, baked potato and butter. | | Uses | Pyruvophenone was used in the synthesis of opioid receptor agonists for gastrointestinal disorders. The enantioselective hydrogenation of Pyruvophenone over Pt colloids was also studied. | | Definition | ChEBI: An alpha-diketone that consists of 1-phenylpropane bearing keto substituents at positions 1 and 2. It is found in coffee. | | Aroma threshold values | Aroma characteristics at 1.0%: creamy, buttery, fatty, slightly vanillalike, slightly walnut nutty, almond
and marzipanlike with a woody styraxlike nuance, and having a slightly sour yeasty and fermented nuance. | | Taste threshold values | Taste characteristics at 5 ppm: cultured creamy, dairylike and slightly astringent with a slight spicy caraway/
cumin nuance. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 36, p. 3553, 1971 DOI: 10.1021/jo00822a019 | | General Description | 1-Phenyl-1,2-propanedione is a volatile flavor compound found in khat leaves and cambará honey. | | Synthesis | By oxidation of propiophenone or benzyl methyl ketone with selenium dioxide; by the acid hydrolysis of oximonopropiophenone
or other synthetic routes. |
| 1-Phenyl-1,2-propanedione Preparation Products And Raw materials |
|