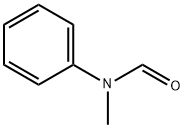
Other grades of this product :
| N-Methylformanilide Basic information |
| N-Methylformanilide Chemical Properties |
| Melting point | 8-13 °C (lit.) | | Boiling point | 243-244 °C (lit.) | | density | 1.095 g/mL at 25 °C (lit.) | | vapor pressure | 2.66 Pa (25 °C) | | refractive index | n20/D 1.561(lit.) | | Fp | 260 °F | | storage temp. | Store below +30°C. | | solubility | 10.3g/l | | pka | 0.53±0.50(Predicted) | | form | Liquid | | color | Clear colorless to yellow | | PH | 3.5-5.5 (H2O, 20℃)(saturated aqueous solution) | | Water Solubility | immiscible | | BRN | 636496 | | LogP | 1.41 at 25℃ | | CAS DataBase Reference | 93-61-8(CAS DataBase Reference) | | NIST Chemistry Reference | Formamide, N-methyl-N-phenyl-(93-61-8) | | EPA Substance Registry System | Formamide, N-methyl-N-phenyl- (93-61-8) |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-43-38 | | Safety Statements | 36/37 | | WGK Germany | 2 | | TSCA | Yes | | HS Code | 29242995 |
| N-Methylformanilide Usage And Synthesis |
| Chemical Properties | clear colourless to yellow liquid | | Uses | Organic synthesis. | | Uses | N-Methylformanilide is used as a formylating reagent for certain organometallics. It is in combination with phosphoryl chloride used for Vilsmeier-Haack reactions and in heterocycle syntheses. Further, it acts as a swelling agent in the dyeing process of meta-aramid fibers. | | Flammability and Explosibility | Notclassified |
| N-Methylformanilide Preparation Products And Raw materials |
| Raw materials | Potassium hydroxide solution-->FORMANILIDE-->carbanilic acid | | Preparation Products | Buprofezin-->O-(2,3,4,5,6-PENTAFLUOROBENZYL)HYDROXYLAMINE HYDROCHLORIDE-->3-(CYANOMETHYL)-2,4,5-TRIMETHYLTHIOPHENE-->2,3,5-Trimethylthiophene-->3-IODO-2,4,5-TRIMETHYLTHIOPHENE-->1-METHYL-1,2,3,4-TETRAHYDRO-QUINOLINE-6-CARBALDEHYDE-->5-Ethyl-2-thiophenecarboxaldehyde-->3-METHYLBENZO[B]THIOPHENE-2-CARBOXALDEHYDE-->5-Bromothiophene-2-carbaldehyde-->4,5-DIMETHYLTHIOPHENE-2-CARBOXALDEHYDE-->1-Pyrenecarboxaldehyde-->5-DIMETHYLAMINO-THIOPHENE-2-CARBALDEHYDE-->N-CHLOROMETHYL-N-PHENYLCARBAMOYL CHLORIDE-->2,4,6-Trimethoxybenzaldehyde-->Benzyl formate-->2-Chloro-5-thiophenecarboxaldehyde-->5-Methylthiophene-2-carboxaldehyde-->2-(Trifluoromethyl)benzaldehyde-->2,5-DIMETHOXY-4-METHYLBENZALDEHYDE-->2,3,4,5-Tetrafluorobenzaldehyde |
|