Other grades of this product :
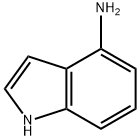
| 4-Aminoindole Basic information |
| 4-Aminoindole Chemical Properties |
| Melting point | 106-109 °C (lit.) | | Boiling point | 354.0±15.0 °C(Predicted) | | density | 1.268±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | pka | 18.23±0.30(Predicted) | | form | powder to crystal | | color | White to Gray to Brown | | Water Solubility | Insoluble | | Sensitive | Air Sensitive | | BRN | 114919 | | CAS DataBase Reference | 5192-23-4(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36-24/25 | | WGK Germany | 3 | | F | 8-10-34 | | HazardClass | AIR SENSITIVE, IRRITANT-HARMFUL, KEEP COLD | | HS Code | 29339990 |
| 4-Aminoindole Usage And Synthesis |
| Chemical Properties | Greenish-grey to tan powder | | Uses | Reactant for preparation of:• ;Inhibitors of bacterial thymidylate synthase1• ;Mimetics of non-alkaloid toxin lignan anticancer and antiviral agent Podophyllotoxin (PPT)2• ;Inhibitors of Gli1-mediated transcription in the Hedgehog pathway3• ;Protein kinase C θ (PKCθ) inhibitors4• ;Indolic non-peptidic HIV protease inhibitors5• ;Transient receptor potential cation channel subfamily V member 1 (TRPV1) antagonists6• ;Cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors7• ;11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) inhibi | | Uses | 4-Aminoindole may be used to synthesize:- macrolactam tumour promoter indolactam V
- tricyclic structure of 2-substituted-pyrrolo[2,3-h]quinolin-4-one
- 4-azidoindole
| | Synthesis Reference(s) | The Journal of Organic Chemistry, 48, p. 5130, 1983 DOI: 10.1021/jo00173a071 | | General Description | 4-Aminoindole is an indole derivative. Its cytokinin activity has been assessed by tobacco pith callus bioassay. 4-Aminoindole can be prepared by reacting 2,6-dinitrotoluene and N,N-dimethylformamide dimethylacetal in anhydrous DMF. |
| 4-Aminoindole Preparation Products And Raw materials |
|