Other grades of this product :
| 5-METHYL-3-HEPTANONE Basic information |
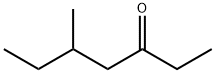
| Product Name: | 5-METHYL-3-HEPTANONE | | Synonyms: | Ethylamylketone~Ethyl2-methylbutylketone;5-Methyl-3-heptanone,96%;METHYL-3-HEPTANONE, 5-(SG);5-METHYL-3-HEPTANONE WITH GC;HEPTAN-3-ON, 5-METHYL;Amyl Ethyl Ketone
Ethyl Amyl Ketone;2-methylbutylethylketone;3-Heptanone,5-methyl- | | CAS: | 541-85-5 | | MF: | C8H16O | | MW: | 128.21 | | EINECS: | 208-793-7 | | Product Categories: | | Mol File: | 541-85-5.mol |
| 5-METHYL-3-HEPTANONE Chemical Properties |
| Melting point | -56.65°C | | Boiling point | 157-162 °C (lit.) | | density | 0.823 g/mL at 25 °C (lit.) | | vapor pressure | 2.7 hPa (25 °C) | | refractive index | n20/D 1.414(lit.) | | Fp | 111 °F | | storage temp. | Store below +30°C. | | solubility | 3g/l | | form | Colorless liquid | | color | Colorless liquid with a fruity odor | | explosive limit | 0.9-48%(V) | | Water Solubility | 1.92g/L at 19.9℃ | | Merck | 14,6084 | | BRN | 506345 | | Henry's Law Constant | 1.30 x 10-4 atm?m3/mol at 20 °C (approximate - calculated from water solubility and vapor pressure) | | Exposure limits | TLV-TWA 130 mg/m3 (25 ppm) (ACGIH). | | LogP | 2.147 at 25℃ | | CAS DataBase Reference | 541-85-5(CAS DataBase Reference) | | EPA Substance Registry System | 5-Methyl-3-heptanone (541-85-5) |
| Hazard Codes | Xi | | Risk Statements | 10-36/37 | | Safety Statements | 23 | | RIDADR | UN 2271 3/PG 3 | | WGK Germany | 1 | | RTECS | MJ7350000 | | HS Code | 2914 19 90 | | HazardClass | 3.2 | | PackingGroup | III | | Toxicity | LD50 orally in Rabbit: 3500 mg/kg LD50 dermal Rabbit > 16000 mg/kg | | IDLA | 100 ppm |
| 5-METHYL-3-HEPTANONE Usage And Synthesis |
| Chemical Properties | CLEAR LIGHT YELLOW LIQUID | | Chemical Properties | 5-Methyl-3-heptanone is a colorless liquid of low volatility.
It has an agreeable penetrating odor that resembles the
essence of apricots and peaches. Threshold odor concentrations
of 6 and ,5 ppm have been reported . | | Uses | Solvent for resins; organic intermediate | | Uses | - 5-Methyl-3-heptanone can be converted into the corresponding gem-dihydroperoxide using aqueous hydrogen peroxide and chlorosulfonic acid as a catalyst at room temperature.
- It can be reduced to its corresponding alcohol using sodium borohydride in urea/choline chloride eutectic salt.
- It can undergo condensation with tert-butyl-sulfinamide to form the corresponding chiral tert-butyl-sulfinyl-ketimine, which can further react with ylides derived from trimethylsulfonium iodide and S-allyl tetrahydrothiophenium bromide to form highly substituted chiral aziridines.
| | Uses | Ethyl amyl ketone is used as a solvent forvinyl resins and nitrocellulose resins. | | Production Methods | U.S. production and importation of 5-methyl-2-heptanone
was estimated to be relatively low (<25,000 lb at a single
site) in 2005 as data for 5-methyl-2-heptanone were not
included in the 2006 U.S. EPA Inventory Update Reporting
database. | | Health Hazard | Exposure to ethyl amyl ketone can causeirritation of the eyes, nose, and skin. Athigh concentrations its exposure can leadto ataxia, prostration, respiratory pain, andnarcosis. A 5-minute exposure to 50 ppmmay produce a mild irritating effect on theeyes and nose in humans. Inhalation of3000 ppm of ethyl amyl ketone for 4 hourswas highly toxic to mice, and 6000 ppm for8 hours was lethal to rats. The odor thresholdis 6 ppm. | | Fire Hazard | Combustible liquid; flash point (open cup)
59°C (138°F); vapor density 2 torr at 25°C
(77°F); fire-extinguishing agent: “alcohol”
foam, dry chemical, or CO2; a water spray
may be effective to cool it below its flash
point. It forms explosive mixtures with air
at elevated temperatures; the LEL and UEL
values are not reported. | | Flammability and Explosibility | Flammable | | Safety Profile | Moderately irritating to
skin, eyes, and mucous membranes by
inhalation and ingestion. Narcotic in high
concentration. Flammable liquid when
exposed to heat, sparks, or flame. When
heated to decomposition it emits acrid
smoke. To fight fire, use foam, CO2, dry
chemical. See also KETONES. | | Environmental fate | Chemical/Physical. 5-Methyl-3-heptanone will not hydrolyze because it does not contain a
hydrolyzable functional group. | | Waste Disposal | Ethyl amyl ketone wastes can be destroyedby incineration or by treatment with moltenmetal salts. |
| 5-METHYL-3-HEPTANONE Preparation Products And Raw materials |
|