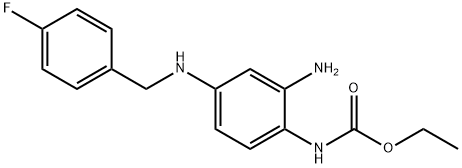
| Description | Retigabine was approved in March 2011 by the European Commission
for the adjunctive treatment of partial-onset seizures in adults who have
epilepsy; in June 2011, the U.S. FDA approved the same drug, known in
the United States as ezogabine.
Retigabine differs from all currently approved antiepileptic drugs in that it acts as a
selective positive allosteric modulator (opener) of KCNQ2-5 potassium
channels, which are key regulators of neuronal excitability. The
discovery of retigabine was based on modification of an analgesic agent
flupirtine that had serendipitously shown potent anticonvulsant activity in
animal models of epilepsy. Changing a central 2,3,6-triaminopyridine to a
1,2,4-triaminobenzene decreased analgesic activity while enhancing
antiepileptic activity. Retigabine (D-23129) was shown to be a broad
spectrum anticonvulsant with oral activity in a variety of animal models.
The mechanism of action of retigabine was discovered well after its
in vivo activity was recognized. Retigabine can be prepared by
reductive amination of 2-ethoxycarbonylamino-5-(4-fluorobenzylamino)-
nitrobenzene with 4-fluorobenzaldehyde, followed by hydrogenation in
the presence of Raney nickel. |
| Description | Retigabine (Item No. 21449) is an analytical reference standard categorized as a positive allosteric modulator (PAM) of KCNQ2-5 (Kv7.2-7.5) potassium ion channels. Formulations containing retigabine have been reported to induce euphoria. Retigabine is regulated as a Schedule V compound in the United States. This product is intended for research and forensic applications. |
| Chemical Properties | Purple Solid |
| Originator | ASTA Medica group (Germany) |
| Uses | Retigabine may be used as a reference standard in the determination of retigabine in biological matrices using high-performance liquid chromatography coupled with tandem mass spectrometry with positive ion atmospheric pressure chemical ionization (HPLC-APCI-MS/MS). |
| Uses | A new experimental anticonvulsant drug. Anxiolytic. |
| Definition | ChEBI: A substituted aniline that is benzene-1,2,4-triamine bearing ethoxycarbonyl and 4-fluorobenzyl substituents at positions N-1 and N-4 respectively. An anticonvulsant used to treat seizures associated with epilepsy in adults. |
| Brand name | Trobalt (European Union), Potiga |
| General Description | Retigabine is an antiepileptic drug, which activates?neuronal KCNQ-type K+ channels by inducing a large hyperpolarizing shift of steady-state?activation. |
| Biochem/physiol Actions | Retigabine (Ezogabine) is a Kv7.2-7.5 (KCNQ2-5) neuronal potassium channel opener witrh anticonvulsant activity. |
| Mechanism of action | Retigabine has a novel mechanism of action for an antiseizure drug, acting as positive allosteric modulator of the neuronal potassium channels KNCQ (Kv2 to 5). Under normal physiologic conditions, KNCQ channels help establish the neuronal resting membrane potential, by providing a continual hyperpolarizing influence. |
| Pharmacology | Retigabine is quickly absorbed, and reaches maximum plasma concentrations between half an hour and 2 hours after a single oral dose. It has a moderately high oral bioavailability (50–60%), a high volume of distribution (6.2 L/kg), and a terminal half-life of 8 to 11 hours. Retigabine requires thrice-daily dosing due to its short half-life.Retigabine is metabolized in the liver, by N-glucuronidation and acetylation. The cytochrome P450 system is not involved. Retigabine and its metabolites are excreted almost completely (84%) by the kidneys. |
| Clinical Use | Retigabine is an investigational antiepileptic drug with a novel mechanism of action that involves opening of neuronal voltageactivated K+
channels that serves to stabilize the membrane potential and to control neuronal excitability. Thus, retigabine also
may prove to be useful in the treatment of other diseases associated with neuronal hyperexcitability. |
| Synthesis | Commercially available 4-fluorobenzaldehyde (188) was condensed
with 4-amino-2-nitroaniline (189) and the resulting imine
was reduced with sodium borohydride to give aniline 190 in 79–85% yield. Aniline 190 was acylated with
diethylcarbonate (191) to give nitrobenzene 192 in 80–88% yield.
Reduction of the nitro group via catalytic hydrogenation provided
retigabine/ezogabine (XVIII) in 70–90% yield. An alternative method
(not shown) involved initial reduction of nitrobenzene 190 with
Zn/NH4Cl followed by acylation with diethylcarbonate (191) or ethyl chloroformate/Hunig’s base, providing retigabine/ezogabine
(XVIII) in 46–81% overall yield. |