Other grades of this product :
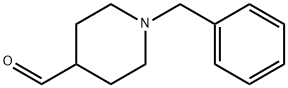
| N-Benzylpiperidine-4-carboxaldehyde Basic information | | Synthesis |
| N-Benzylpiperidine-4-carboxaldehyde Chemical Properties |
| Melting point | 145-149 °C | | Boiling point | 315.4 °C/760 mmHg | | density | 1.026 g/mL at 25 °C | | refractive index | n20/D 1.537 | | Fp | >230 °F | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | Acetone (Slightly), Chloroform (Slightly), Dichloromethane (Slightly), DMSO (Sli | | form | Liquid | | pka | 8.04±0.10(Predicted) | | color | Clear colorless to pale yellow | | Sensitive | Air Sensitive | | InChIKey | SGIBOXBBPQRZDM-UHFFFAOYSA-N | | CAS DataBase Reference | 22065-85-6(CAS DataBase Reference) |
| Hazard Codes | T | | Risk Statements | 25-41 | | Safety Statements | 26-28-36/37/39 | | RIDADR | UN 2810 6.1/PG 3 | | WGK Germany | 3 | | HazardClass | IRRITANT | | PackingGroup | Ⅲ | | HS Code | 29333990 |
| N-Benzylpiperidine-4-carboxaldehyde Usage And Synthesis |
| Synthesis | A round
bottom flask was charged with oxalyl chloride (16.2 g, 0.12
mol), dichloromethane (150 mL) and anhydrous dimethyl sulfoxide
(20 mL). Stir the reaction mixture mass in a cryo bath at -70 °C
for 15 min. The resulting mixture is charged dropwise with N-benzyl piperidine alcohol 3 (20 g, 0.097 mol), along with
triethylamine (24.6 g, 0.24 mol) and continued stirring for the
next 15 min. After that, the mass is allowed to attain room
temperature overnight, then diluted with dichloromethane (100
mL) and quenched with cold water. The organic layer was
washed subsequently with 5 % HCl solution, brine solution, 5
% sodium bicarbonate solution and dried over sodium sulfate.
Toluene was removed in vacuo to afford N-Benzylpiperidine-4-carboxaldehyde (19.2 g, 96 %). | | Chemical Properties | Colorless Oil | | Uses | Reactant for:- Stereospecific allylic alkylation
- Reactions of Grignard reagents with carbonyl compounds
- Fluorodenitrations and nitrodehalogenations for labeled PET ligands and fluoropharmaceuticals
- Selective α1 receptor antagonists
Reactant for synthesis of:- Donepezil
- MCH-R1 antagonists
|
| N-Benzylpiperidine-4-carboxaldehyde Preparation Products And Raw materials |
|