| Description | BAL-8557 (isavuconazonium) is the water-soluble pro-drug of BAL-4815 (isavuconazole). The conversion to isavuconazole is rapid and complete with very little cleavage product detectable – thus this chapter will focus entirely on isavuconazole. Isavuconazole is produced and developed by Basilea Pharmaceutica and, in common with the other azoles, works by inhibition of ergosterol synthesis. Isavuconazole is a water-soluble compound (the only broad-spectrum water soluble triazole) with oral and intravenous formulations and excellent bioavailability. Its long elimination half-life allows up to once-weekly dosing. |
| Uses | Isavuconazole is a new triazole currently undergoing phase III clinical trials. This compound has shown in vitro activity against a large number of clinical important yeasts and molds including Asperg
illus spp., Fusarium spp., Scedosporium spp., Candida spp., the Zygomycetes and Cryptococcus spp. |
| Uses | Isavuconazole is a new triazole currently undergoing phase III clinical trials. This compound has shown in vitro activity against a large number of clinical important yeasts and molds including Aspergillus spp., Fusarium spp., Scedosporium spp., Candida spp., the Zygomycetes and Cryptococcus spp. |
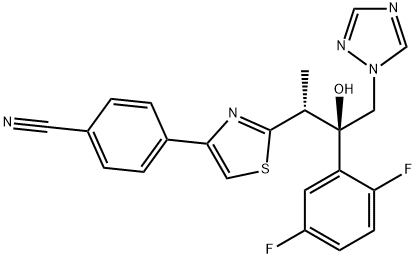
| Definition | ChEBI: A 1,3-thiazole that is butan-2-ol which is substituted at positions 1, 2, and 3 by 1,2,4-triazol-1-yl, 2,5-difluorophenyl, and 4-(p-cyanophenyl)-1,3-thiazol-2-yl groups, respectively. It is an antifungal drug used for the treatment of invasive
aspergillosis and invasive mucormycosis. |
| Antimicrobial activity | Isavuconazole is a new broad-spectrum triazole with activity against Candida, Cryptococcus, Aspergillus, Zygomycetes, Rhizopus, and Rhizomucor spp. and dimorphic fungi including Histoplasma capsulatum and Blastomyces dermatitidis and a range of dermatophytes. In animal models, isavuconazole is highly effective against systemic candidiasis and disseminated Aspergillus fumigatus and flavus infections. |
| Biochem/physiol Actions | Maximum plasma concentrations of BAL 4815 are observed 1.5–3 hours after oral doses or at the end of the 1-hour intravenous infusion. Mean elimination half-lives are remarkably long more than 50 and 70 hours after oral and intravenous doses, respectively, allowing for once-daily (or less frequent) dosing. The volume of distribution is large and systemic clearance low. The protein binding is 98%. Escalation in multiple dosing regimens results in a proportional increase in maximum plasma drug concentration. |
| Clinical Use | Three regimens of isavuconazonium (BAL 8557) – single 200 mg dose followed by 50 mg daily, single 400 mg followed by 100 mg daily and single 400 mg followed by 400 mg weekly –were demonstrated to be as efficacious as fluconazole in a phase II study conducted on patients with uncomplicated esophageal candidiasis. The results of a phase II open-label dose-escalating trial of intravenous isavuconazonium as prophylaxis in AML (NCT00413439) are pending (NIH, 2008). Two phase III randomized double-blind studies to evaluate the safety and efficacy of isavuconazole are currently under way, versus caspofungin followed by voriconazole in the treatment of candidaemia and invasive candidiasis and versus voriconazole in primary treatment of invasive filamentous fungal infection . A phase III open-label study using isavuconazole in patients with aspergillosis and renal impairment and those with rare fungi is being planned NCT00634049. |
| Toxicology | In animals, isavuconazole has revealed no mutagenic, allergenic,
phototoxic, or irritant potential. In
15 healthy volunteers, single doses of isavuconazole (up to 200 mg)
were well tolerated, and no severe or serious adverse events occurred. In a multiple-dose pharmacokinetic
study, 24 healthy males received isavuconazole for 21 days of oral or
14 days of intravenous dosing. The
most frequent adverse events were headache, nasopharyngitis, and
rhinitis, and all were mild or moderate; however one subject on oral
high-dose BAL 8557 had mild transient abnormal liver function on day
14 of therapy, which resolved despite continuing the drug. No clinically relevant changes in vital signs or
electrocardiogram were observed. In a double-blind randomized phase
II trial for the treatment of esophageal candidiasis, BAL 8557 was safe
and well tolerated, with an adverse event profile similar to that of
fluconazole, the comparator drug. |
| Drug interactions | Rifampicin, a potent CYP3A4 inducer, decreases the Cmax and AUC
of isavuconazole . Ketoconazole
increases exposure to isavuconazole.
Neither ciclosporin nor warfarin is affected by isavuconazole co-administration. Isavuconazole increases tacrolimus levels. |
| Metabolism | Molecular weight (daltons) 814.8 (as
isavuconazonium
sulphate)
% Protein binding >99
% Excreted unchanged in urine <1
Volume of distribution (L/kg) 450 Litres
Half-life - normal/ESRF (hrs) 2-4 (as isavuconazole);
80-130 (as
isavuconazonium) /
Unchanged |