Other grades of this product :
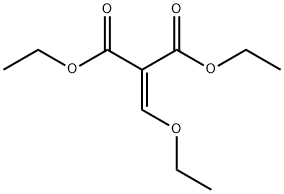
| Diethyl ethoxymethylenemalonate Basic information |
| Diethyl ethoxymethylenemalonate Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 22-36/37/38-42/43 | | Safety Statements | 26-36/37/39-27-24/25 | | WGK Germany | 1 | | RTECS | OO1100000 | | Autoignition Temperature | 215 °C | | TSCA | Yes | | HS Code | 29189090 | | Toxicity | LD50 orally in Rabbit: 925 mg/kg LD50 dermal Rat > 2000 mg/kg |
| Diethyl ethoxymethylenemalonate Usage And Synthesis |
| Chemical Properties | CLEAR COLOURLESS TO LIGHT YELLOW LIQUID | | Uses | Intermediate in the production of Flumequine | | Uses | Diethyl ethoxymethylenemalonate is used for synthesis of pyrido[3,2-e]pyrimido[1,2-c]pyrimidines. Initially, it was used to monitor lysine decarboxylase activity. It is useful in the determination of amino acids by precolumn derivatization by using reversed-phase high-performance liquid chromatography (HPLC). It plays an important role in the Gould-Jacobs reaction to prepare quinolines. For example, anile reacts with this reagent to give 4-hydroxyquinoline. It acts as an intermediate in the production of flumequine, which is a fluoroquinolone antibiotic. | | Safety Profile | Moderately toxic by
ingestion. A skin irritant. A combustible
liquid. When heated to decomposition it
emits acrid smoke and irritating fumes. | | Purification Methods | Likely impurity is diethyl diethoxymethylene malonate which is difficult to separate from diethyl ethoxymethylene malonate by distillation, and it is necessary to follow the course of the distillation by the change in refractive index instead of boiling point. After a low boiling fraction is collected, there is obtained an intermediate fraction (n 20 1.414—1.458), the size of which depends on the amount of the diethoxymethylene compound. This fraction is fractionated through a 5inch Vigreux column (p 11) at low pressure avoiding interruption in heating. Fraction b 108-110o/0.25mm is ca 10o lower than the submitters' fraction (b 97.2o/0.25mm, n D 1.4612—1.4623) [Org Synth Coll Vol III 395 1955, Fuson et al. J Org Chem 11 197 1946, Duff & Kendal J Chem Soc 893 1948]. [Beilstein 3 IV 1192.] |
| Diethyl ethoxymethylenemalonate Preparation Products And Raw materials |
| Raw materials | Triethyl orthoformate-->Diethyl malonate | | Preparation Products | Norfloxacin-->Alachlor-->Lomefloxacin-->Orotic acid-->ETHYL 4-CHLORO-3-METHYLTHIENO[2,3-B]PYRIDINE-5-CARBOXYLATE-->Ethyl 4-chloro-2-(trifluoromethyl)pyrimidine-5-carboxylate-->ETHYL 4-AMINO-2-(METHYLTHIO)PYRIMIDINE-5-CARBOXYLATE-->Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate-->5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one-->Levofloxacin hydrochloride-->7-TRIFLUOROMETHYL-4-QUINOLINETHIOL-->1,4-DIHYDRO-2-(METHYLTHIO)-4-OXO-5-PYRIMIDINE-CARBOXYLATE ACID ETHYL ESTER-->7-BROMO-3,4-DIHYDRO-4-OXOQUINOLINE-3-CARBOXYLIC ACID-->7-(TRIFLUOROMETHYL)-4-QUINOLINOL-->4-HYDROXY-7-TRIFLUOROMETHYL-3-QUINOLINECARBOXYLIC ACID-->Fleroxacin-->4-HYDROXY-2-METHYL-PYRIMIDINE-5-CARBOXYLIC ACID ETHYL ESTER-->Nalidixic acid-->ETHYL 4-CHLOROTHIENO[2,3-B]PYRIDINE-5-CARBOXYLATE-->4-HYDROXY-2-PHENYL-5-PYRIMIDINECARBOXYLIC ACID-->3,4-DIHYDRO-8-HYDROXY-4-OXOQUINOLINE-3-CARBOXYLIC ACID-->Ethyl 7-bromo-3,4-dihydro-4-oxoquinoline-3-carboxylate ,97%-->4-AMINO-2-METHYLSULFANYL-PYRIMIDINE-5-CARBOXYLIC ACID-->4-HYDROXY-8-METHOXYQUINOLINE-3-CARBOXYLIC ACID-->6-CHLORO-[1,2,4]TRIAZOLO[4,3-B]PYRIDAZINE-->ETHYL 4-HYDROXY-7-(TRIFLUOROMETHYL)QUINOLINE-3-CARBOXYLATE-->Ethyl 4-hydroxy-2-phenylpyrimidine-5-carboxylate-->Decoquinate-->4-HYDROXY-8-METHOXY-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER |
|