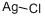Other grades of this product :
| Silver chloride Chemical Properties |
| Melting point | 455 °C (lit.) | | Boiling point | 1550 °C | | density | 5.56 | | vapor pressure | 1 mm Hg ( 912 °C) | | refractive index | 2.071 | | Fp | 1550°C | | storage temp. | Store at +5°C to +30°C. | | solubility | 0.00188g/l | | form | beads | | color | Yellow | | Specific Gravity | 5.56 | | Water Solubility | 1.93 mg/L (25 ºC) | | Sensitive | Light Sensitive | | Merck | 14,8509 | | Solubility Product Constant (Ksp) | pKsp: 9.75 | | Stability: | Stable, but discolours in light. | | InChIKey | HKZLPVFGJNLROG-UHFFFAOYSA-M | | CAS DataBase Reference | 7783-90-6(CAS DataBase Reference) | | NIST Chemistry Reference | Silver chloride(7783-90-6) | | EPA Substance Registry System | Silver chloride (7783-90-6) |
| Hazard Codes | N | | Risk Statements | 50/53-50 | | Safety Statements | 24/25-61-60 | | RIDADR | UN 3077 9 / PGIII | | WGK Germany | 3 | | RTECS | VW3563000 | | F | 8 | | TSCA | Yes | | HS Code | 2843 29 00 | | HazardClass | 9 | | Toxicity | LD50 orally in Rabbit: > 5110 mg/kg |
| Silver chloride Usage And Synthesis |
| Physical Properties | White granular powder or cubic crystals; refractive index 2.071; darkens on exposure to light; density 5.56 g/cm3; Moh’s hardness 2.5; melts at 455°C; vaporizes at 1,547°C; vapor pressure 1 and 5 torr at 912 and 1,019°C; insoluble in water, alcohol and dilute acids; soluble in ammonia solution and concentrated sulfuric acid, alkali cyanide, ammonium carbonate; also soluble in potassium bromide and sodium thiosulfate solutions.
| | Uses | Silver chloride is used in silver plating and to obtain pure silver. The salt also finds applications in photography and optics; in photochromic glass; and in electrodes and batteries. It is used to make antiseptic silver solution. It occurs as the mineral cerargyrite.
| | Preparation | Silver chloride is prepared by slowly adding an alkali metal chloride solution to a hot solution of silver nitrate. The solution mixture is boiled:
Ag+ (aq) + Cl¯ (aq) → AgCl (s)
The precipitate is washed with hot water. The product is purified by dissolving in ammonia solution, filtering out any insoluble residues, and then adding hydrochloric acid to reprecipitate silver chloride. Preparation should be carried out in the dark in ruby red light.
| | Chemical Properties | Silver chloride, AgCl, is a white,granular powder that darkens on exposure to light,finally turning black.It exists in several modifications differing in behavior toward light and solubility in various solvents. Soluble in ammonium hydroxide, concentrated sulfuric acid, and sodium thiosulfate and potassium bromide solutions, very slightly soluble in water, can be melted, cast, and fabricated like a metal. Derived by heating a silver nitrate solution and adding hydrochloric acid or salt solution. The whole is boiled, then filtered. This must take place in the dark or under a ruby-red light. Used in photography,photometry and optics, batteries, photochromic glass,silver plating,production of pure silver, and as an antiseptic. Single crystals are used for infrared absorption cells and lens elements and as a lab reagent
| | Uses | In silver plating, in making antiseptic silver preparations. | | Uses | Found in nature as horn silver, this white powder is made by
the combination of a soluble chloride and silver nitrate. Silver
bromide could also be formed by exposing metallic silver to
the fumes of bromine as in the daguerreotype process. It is
soluble in sodium thiosulfate, potassium bromide solutions,
and strong ammonia. This silver halide was the first to be
observed to darken spontaneously by exposure to light. Silver
chloride formed the basis of the photogenic drawing, salted
paper print, albumen print, collodion-chloride POP, gelatin
chloride POP, and gaslight paper. | | Uses | Used in photographic films, to coat and silver glass,
as an antiseptic, and to absorb infrared light in lenses. | | Uses | Employed in Silver plating. Owing to its characteristic of reversible reduction to silver metal, it is used in photochromic lenses. Used as a cathode in sea water activated batteries. In electrochemistry, silver chloride electrode is used for potentiometric measurements. It serves as an antidote for mercury poisoning, and eliminates mercury from body. It is used in glass manufacturing industry. It is useful in the production of bandages, wound healing products and inglaze lustre, personal deodorant products, as well as for long term preservation of drinking water in water tanks; its pharmaceutical composition finds use as an antibacterial agent. | | Definition | White, granular powder; darkens on
exposure to light, finally turning black. Exists in
several modifications differing in behavior toward
light and in their solubility in various solvents.Soluble in ammonium hydroxide, concentrated sulfuric acid and sodiu | | Hazard | As for silver.
| | Flammability and Explosibility | Nonflammable | | Purification Methods | Recrystallise it from conc NH3 solution by acidifying with HCl, filtering off the solid, washing it with H2O and drying it in a vacuum. It is soluble in NH3 and should be kept in the dark. |
| Silver chloride Preparation Products And Raw materials |
|