Other grades of this product :
| Triethyl borate Basic information |
| Triethyl borate Chemical Properties |
| Melting point | -84.5 °C | | Boiling point | 117-118 °C(lit.) | | density | 0.858 g/mL at 25 °C(lit.) | | vapor density | 5.04 (vs air) | | refractive index | n20/D 1.374(lit.) | | Fp | 52 °F | | storage temp. | 0-6°C
| | solubility | Miscible with alcohol and ether. | | form | Liquid | | Specific Gravity | 0.864 | | color | Colorless | | Water Solubility | decomposes | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | BRN | 1699052 | | CAS DataBase Reference | 150-46-9(CAS DataBase Reference) | | NIST Chemistry Reference | Boric acid, triethyl ester(150-46-9) | | EPA Substance Registry System | Boric acid (H3BO3), triethyl ester (150-46-9) |
| Hazard Codes | F | | Risk Statements | 11 | | Safety Statements | 7-16-23-9-33 | | RIDADR | UN 1176 3/PG 2 | | WGK Germany | 2 | | RTECS | ED5075000 | | F | 21 | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | II | | HS Code | 29209085 | | Toxicity | eye-rbt 100 mg MLD 14KTAK -,706,64 |
| Triethyl borate Usage And Synthesis |
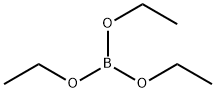
| Chemical Properties | Triethyl borate is a Colorless liquid; mild odor. Hydrolyzes rapidly depositing boric acid in finely divided crystalline form. | | Uses | Triethyl borate is used as a solvent in resins, paints, varnishes, adhesives and sealant chemicals. It is used as a catalyst of synthetic waxes and resins. It is an active component of flame retardants in the textile industry and in welding fluxes. It is used to prepare m-tolyl-acetic acid ethyl ester by reacting with 1-bromomethyl-3-methyl-benzene and carbon monoxide using as1,5-hexadiene?dium chloride as a catalyst. Further, it is used in pyrotechnics. | | Uses | Triethyl borate may be used as boron source to synthesize lithium borophosphates, alumina borosilicate ceramic nanofibres and boron-doped single wall carbon nanotubes. | | Uses | Antiseptics, disinfectants, antiknock agent.
Triethyl borate is used as a solvent in resins, paints, varnishes, adhesives and sealant chemicals. It is used as a catalyst of synthetic waxes and resins. It is an active component of flame retardants in the textile industry and in welding fluxes. It is used to prepare m-tolyl-acetic acid ethyl ester by reacting with 1-bromomethyl-3-methyl-benzene and carbon monoxide using as 1,5-hexadiene dium chloride as a catalyst. Further, it is used in pyrotechnics.
Triethyl borate may be used as boron source to synthesize lithium borophosphates, alumina borosilicate ceramic nanofibres and boron-doped single wall carbon nanotubes.CVD precursor for boron modified SiO2 in microelectronics Intermediate for organic-modified borosiloxanes.
| | Preparation | Triethyl borate can be prepared by adding anhydrous ethanol with boric acid or boron trioxide. Can be purified via distillation. | | Definition | ChEBI: A member of the class of borate esters obtained by the formal condensation of three equivalents of ethanol with boric acid. | | Hazard | Flammable, dangerous fire risk. | | Safety Profile | Moderately toxic by ingestion. Aneye irritant. A very dangerous fire hazard when exposed toheat or flame. When heated to decomposition it emitsacrid smoke and irritating fumes. | | Purification Methods | Dry it over sodium, then distil it. Also fractionate it through a gauze packed column. [Charnlet et al. J Chem Soc 2288 1952, as for tributyl borate Johnson & Tompkins Org Synth Coll Vol II 106 1943, Beilstein 1 III 1339, 1 IV 1365.] |
| Triethyl borate Preparation Products And Raw materials |
|