Other grades of this product :
| Ampiroxicam Basic information |
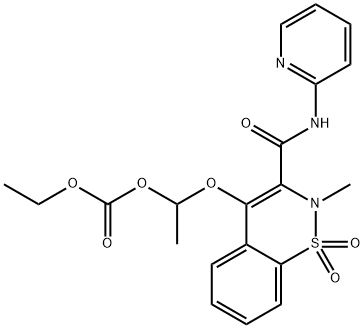
| Product Name: | Ampiroxicam | | Synonyms: | 4-[1-(ethoxycarbonyloxy)ethoxy]-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazine-3-carboxamide-1,1-dioxide;AMPIROXICAM;2H-1,2-Benzothiazine, carbonic acid deriv.;Carbonic acid, ethyl 1-[[2-methyl-1,1-dioxido-3-[(2-pyridinylamino)carbonyl]-2H-1,2-benzothiazin-4-yl]oxy]ethyl ester;Carbonic acid, ethyl 1-[[2-methyl-3-[(2-pyridinylamino)carbonyl]-2H-1,2-benzothiazin-4-yl]oxy]ethyl ester, S,S-dioxide;CP 65703;Flucam;Nasil | | CAS: | 99464-64-9 | | MF: | C20H21N3O7S | | MW: | 447.46 | | EINECS: | 807-877-6 | | Product Categories: | API;FLUCAM;Activators;Inhibitors | | Mol File: | 99464-64-9.mol |
| Ampiroxicam Chemical Properties |
| Melting point | 159-161?C | | density | 1.44±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 12.74±0.20(Predicted) | | color | White to Off-White | | CAS DataBase Reference | 99464-64-9(CAS DataBase Reference) |
| Toxicity | LD50 orally in male, female rats: 1798, 747 mg/kg (Iijima) |
| Ampiroxicam Usage And Synthesis |
| Description | Ampiroxicam is a once-daily non-steroidal anti-inflammatory drug (NSAID)
introduced in Japan. As a non-acidic ether carbonate prodrug of the widely used
antiinflammatory agent, piroxicam, ampiroxicam is similar or more potent than
piroxicam in suppressing carrageenan edema, ultraviolet erythema, traumatic edema,
capillary permeability, acetic acid writhing, yeast algesia and yeast hyperthermia in
many animals. Eioavailability studies in man show undetectable plasma levels of
ampiroxicam indicating 100% conversion to piroxicam during absorption process.
Ampiroxicam has been reported to have fewer side effects on the gastric mucosa
than piroxicam. | | Chemical Properties | White Solid | | Originator | Pflzer (Japan) | | Uses | Prodrug of Piroxicam. Anti-inflammatory. | | Uses | antiinflammatory, analgesic | | Definition | ChEBI: A benzothiazine that is the 1-[(ethoxycarbonyl)oxy]ethyl ether of piroxicam. A prodrug for piroxicam, it is used for the relief of pain and inflammation in musculoskeletal disorders such as rheumatoid arthritis and osteoarthritis. | | Manufacturing Process | To a round bottomed flask equipped with a reflux condenser and stirring bar
were added 2-methyl-N-(2-pyridyl)-4-hydroxy-2H-1,2-benzodiazine-3-
carboxamide 1,1-dioxide (piroxicam, 10.0 g, 30.2 mmol), potassium
carbonate (8.35 g, 60.4 mmol), α-chloroethyl ethyl carbonate (12.35 mL,
13.81 g, 90.6 mmol) and acetone (350 mL). The heterogenous reaction
mixture was heated to reflux under a nitrogen atmosphere. After 19 hours,
anhydrous sodium iodide (22.6 g, 150.7 mmol) was added and reflux
continued for an additional 5 hours. The acetone was removed in vacuum
leaving a brown residue which was treated with water (250 mL) and
methylene chloride (250 mL). The organic layer was separated and the
aqueous layer extracted with additional methylene chloride (250 mL). The
combined organic extracts were washed with water (250 mL), brine (250 mL),
dried (Na2SO4) and concentrated in vacuum to a brown oil. Column
chromatography on silica gel (1:9 ethyl acetate:methylene chloride) afforded
a pale yellow foam (10.67 g, 79.0%). The 4-[1-(ethoxycarbonyloxy)ethoxy]-
2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide was
recrystallized from toluene to give 9.50 g of pure white crystals; melting point
159-161°C. | | Brand name | Nacyl; Flucam | | Therapeutic Function | Antiinflammatory |
| Ampiroxicam Preparation Products And Raw materials |
|