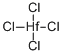Other grades of this product :
| Hafnium(IV) chloride Basic information |
| Hafnium(IV) chloride Chemical Properties |
| Melting point | 432 °C (lit.) | | Boiling point | 315.47°C (estimate) | | density | 1.89 g/cm3 | | vapor pressure | 1 mm Hg ( 190 °C) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Soluble in methanol and acetone. | | form | Powder | | color | White | | Water Solubility | Soluble in water, methanol and acetone. | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | Sensitive | Moisture Sensitive | | Merck | 14,4588 | | Exposure limits | ACGIH: TWA 0.5 mg/m3NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 | | InChIKey | PDPJQWYGJJBYLF-UHFFFAOYSA-J | | CAS DataBase Reference | 13499-05-3(CAS DataBase Reference) | | EPA Substance Registry System | Hafnium chloride (HfCl4), (T-4)- (13499-05-3) |
| Hazard Codes | C | | Risk Statements | 34 | | Safety Statements | 26-36/37/39-45 | | RIDADR | UN 3260 8/PG 2 | | WGK Germany | 3 | | F | 9-21 | | TSCA | Yes | | HazardClass | 8 | | PackingGroup | II | | HS Code | 28273985 |
| Hafnium(IV) chloride Usage And Synthesis |
| Chemical Properties | Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst. | | Physical properties | White monoclinic crystal; sublimes at 317°C; melts at 432°C at 33 atm (triple point); critical temperature 452.5°C; critical pressure 53.49 atm; critical volume 314 cm3/mol. | | Uses | Hafnium tetrachloride is an important intermediate in production of hafnium metal. It also is used to prepare many hafnium compounds. | | Uses | Hafnium chloride tetrahydrofuran complex is an efficient catalyst for the Diels-Alder cycloadditions, Ziegler-Natta polymerization of alkenes. It is used as control rods in nuclear reactors and in manufacture of light bulb filaments. | | Uses | Hafnium (IV) chloride can be used to catalyze the direct ester condensation of carboxylic acids with alcohols effectively. | | Preparation |
Hafnium(IV) chloride can be prepared (i) by chlorination of hafnium dioxide in the presence of carbon: HfO2 + 2Cl2 + C → HfCl4 + 2CO It also may be prepared by several other methods, such as (ii) reaction of carbon tetrachloride with hafnium dioxide above 450°C; (iii) heating a mixture of hafnium dioxide and carbon above 700°C; and (iv) reaction of chlorine with hafnium at elevated temperatures.
| | Flammability and Explosibility | Nonflammable | | Solubility in organics | Soluble in methanol, acetone, reacts strongly with water, highly hygroscopic, hydrolyzes in water. |
| Hafnium(IV) chloride Preparation Products And Raw materials |
|