Other grades of this product :
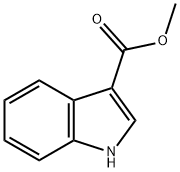
| Methyl indole-3-carboxylate Basic information |
| Methyl indole-3-carboxylate Chemical Properties |
| Melting point | 149-152 °C (lit.) | | Boiling point | 306.47°C (rough estimate) | | density | 1.1999 (rough estimate) | | refractive index | 1.5060 (estimate) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 15.42±0.30(Predicted) | | color | Light Pink | | Water Solubility | Slightly soluble methanol and dimethyl sulfoxide. Insoluble in water. | | BRN | 142023 | | CAS DataBase Reference | 942-24-5(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36-37/39 | | WGK Germany | 3 | | HazardClass | IRRITANT | | HS Code | 29339900 |
| Methyl indole-3-carboxylate Usage And Synthesis |
| Chemical Properties | light brown amorphous powder | | Uses | Methyl indole-3-carboxylate is a reagent in the preaparation of oncrasin-1 derivatives which is a novel inhibitor of the C-terminal domain of RNA polymerase II. | | Uses | Reactant for preparation of:- Nitric oxide synthase (nNOS) inhibitorS
- Protein kinase c alpha (PKCα) inhibitors
- Inhibitors of the C-terminal domain of RNA polymerase II as antitumor agents
- Kinase insert domain receptor (KDR) inhibitors
- Organocatalysts for the anti-Mannich reaction
- Very late antigen-4 (VLA-4) antagonists
- Inhibitors of Human 5-Lipoxygenase
- Serotonin 5-HT4 receptor antagonists
- Hyaluronidase inhibitors
Reactant for:- Enantioselective Meerwein-Eschenmoser Claisen rearrangement reactions?
| | General Description | Methyl indole-3-carboxylate (3-Methoxycarbonylindole, 3-Carbomethoxyindole, Methyl indolyl-3-carboxylate) has been extracted from the marine Streptomyces sp. 060524. Its crystal structure indicates the presence of inter-molecular N-H…O hydrogen bond. It is also obtained during the isolation of sorazinones A and B from Sorangium cellulosum strain Soce895. It undergoes regioselective dibromination with bromine in acetic acid to afford methyl 5,6-dibromoindole-3-carboxylate. |
| Methyl indole-3-carboxylate Preparation Products And Raw materials |
|