Other grades of this product :
| 5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite Basic information |
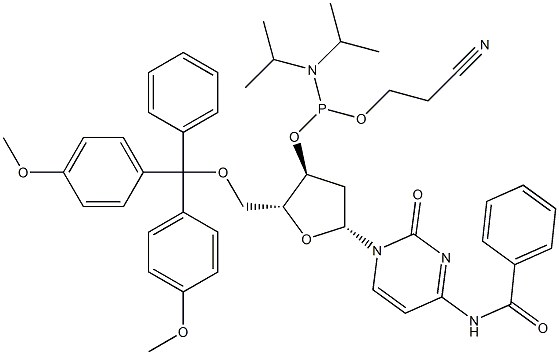
| Product Name: | 5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite | | Synonyms: | REF DUPL: DMT-dC(bz) Phosphoramidite;Bz-dC Phosphoramidite;N4-Benzoyl-2'-deoxy-5'-O-DMT-cytidine 3'-CE phosphoramidite ,98%;N4-Benzoyl-2'-deoxy-5'-O-DMT-cytidine 3'-CE phosphoramidite;2'-DEOXYCYTIDINE PHOSPHORAMIDITE;DNAC-PHOSPHORAMIDITE;dC (N-Bz) Phosphoramidite;N-blocked-5'-O-DMT 3'-CED deoxycytidine phosphoramidite | | CAS: | 102212-98-6 | | MF: | C46H52N5O8P | | MW: | 833.91 | | EINECS: | 801-520-8 | | Product Categories: | Pharmaceutical | | Mol File: | 102212-98-6.mol |
| 5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite Chemical Properties |
| Melting point | >84°C (dec.) | | density | 1.23 at 20℃ | | vapor pressure | 0-0Pa at 20-50℃ | | storage temp. | Sealed in dry,2-8°C | | solubility | Chloroform, DMSO, Methanol (Slightly) | | form | powder or granules | | pka | 8.65±0.20(Predicted) | | color | white to off-white | | InChIKey | PGTNFMKLGRFZDX-SALLYJDFSA-N | | LogP | 6.5 at 20℃ |
| Safety Statements | 24/25 | | WGK Germany | 3 | | HS Code | 29349990 |
| 5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite Usage And Synthesis |
| Uses | 5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite is used to prepare antisense oligonucleotides containing conformationally constrained methoxyaminomethylene and aminooxymethylene and aminomethylene bridged nucleoside analogs. | | Synthesis | These Examples illustrate the phosphitylation of several protected nucleoside reagents with 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite in the presence of several activators according to the present invention. Eleven phosphitylation reactions (1-11) comprising reacting a protected nucleoside reagent with 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite in the presence of an acid-base activator according to the present invention were conducted, and the product yields of each calculated, as described in the General Procedure, below. The various combinations of protected nucleoside, activator base, activator acid, solvent, and yield for each of the 11 reactions are listed in Table 1. General Procedure: The activator base (1.1 to 1.2 equivalents) is added to the solvent and 0.95 to 1.1 equivalents of activator acid is subsequently added thereto at ambient temperature to form the activator solution. About 1 equivalent of the protected nucleoside is dissolved in about 10 equivalents of the solvent in a separate vessel and about 3 equivalents of the solvent is then distilled off under reduced pressure. About 1 to 1.2 equivalents of 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite is added to the nucleoside mixture at ambient temperature, and the activator solution prepared previously is then added to the nucleoside mixture at ambient temperature with vigorous stirring. After 12 hours, the reaction mixture is diluted with toluene and washed with water. The organic layer is separated, dried over sodium sulfate if necessary, and concentrated under reduced pressure. The yield of the desired amidite is then calculated using HPLC techniques, that is, the resulting product mixture is run through an HPLC column using an appropriate eluent, and the area under the HPLC peaks used to determine the %yield of product in the mixture. |
| 5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite Preparation Products And Raw materials |
|