Other grades of this product :
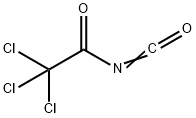
| Trichloroacetyl isocyanate Basic information |
| Trichloroacetyl isocyanate Chemical Properties |
| Melting point | 135-140 °C | | Boiling point | 80-85 °C/20 mmHg (lit.) | | density | 1.581 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.480(lit.) | | Fp | 150 °F | | storage temp. | 2-8°C | | solubility | Miscible with dichloromethane, ether, terahydrofuran and protic solvents. | | form | Liquid | | color | Clear colorless to slightly yellow | | Water Solubility | Decomposition | | Sensitive | Moisture Sensitive | | BRN | 971201 | | InChIKey | GRNOZCCBOFGDCL-UHFFFAOYSA-N | | CAS DataBase Reference | 3019-71-4(CAS DataBase Reference) | | NIST Chemistry Reference | Trichloroacetyl isocyanate(3019-71-4) | | EPA Substance Registry System | Acetyl isocyanate, trichloro- (3019-71-4) |
| Trichloroacetyl isocyanate Usage And Synthesis |
| Chemical Properties | clear colorless to slightly yellow liquid | | Uses | Trichloroacetyl isocyanate was used in catalytic one-pot dehydrative glycosylation of 1-hydroxy carbohydrate. It was also used as a reagent for the conversion of alcohols to carbamates. | | Application | Trichloroacetyl Isocyanate is used as an in situ derivatizing reagent for characterization of alcohols, glycols, phenols, and amines by nuclear magnetic resonance (NMR).It has been reported in the literature that it can be used for the preparation of cefuroxime axetil. | | Preparation | Trichloroacetyl isocyanate can be obtained by reacting 2,2,2-trichloroacetamide with oxalyl chloride. Procedure: A reaction mixture of 2,2,2-trichloroacetamide (100 g, 616 mmol, 1.0 eq) in (COCl)2 (725 g, 5.71 mol, 500 mL, 9.28 eq) was heated to 70°C for 24 hours. The reaction mixture was concentrated under vacuum. To the mixture was added 1,2,4-trichlorobenzene (500 mL) and (COCl)2 (116 g, 914 mmol, 80 mL, 1.48 eq). The reaction mixture was stirred at 100°C for 5 hours. Heat to 125°C for 15 hours. The mixture was then heated to 140°C for 5 hours. The reaction mixture was distilled under water pump (72°C-76°C fractions) to give trichloroacetyl isocyanate (60 g, 319 mmol, 52% yield) as a colorless oil. | | General Description | Conformational stability and vibrational IR and Raman spectra of trichloroacetyl isocyanate has been investigated. Trichloroacetyl isocyanate is commonly employed as in situ derivatizing reagent for the 13C NMR studies of alcohols, phenols and amines. It undergoes 1,5-cycloaddition reaction with anhydro-2-deoxy-D-erythro- and -L-threo-pent-1-enitols and 1,5-anhydro-2-deoxy-D- and -L-arabino-hex-1-enitols to yield [2+2] and [4+2] cycloadducts. |
| Trichloroacetyl isocyanate Preparation Products And Raw materials |
|