Other grades of this product :
| Indoline Basic information |
| Indoline Chemical Properties |
| Melting point | -21 °C | | Boiling point | 220-221 °C (lit.) | | density | 1.063 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.592(lit.) | | Fp | 199 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | 5g/l | | pka | 5.20±0.20(Predicted) | | form | liquid (clear) | | color | clear dark brown | | Water Solubility | 5 g/L (20 ºC) | | Sensitive | Light Sensitive | | BRN | 111915 | | CAS DataBase Reference | 496-15-1(CAS DataBase Reference) | | NIST Chemistry Reference | 1H-Indole, 2,3-dihydro-(496-15-1) | | EPA Substance Registry System | 1H-Indole, 2,3-dihydro- (496-15-1) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 23-24/25-37/39-26 | | WGK Germany | 3 | | RTECS | NL6906300 | | Hazard Note | Irritant | | TSCA | Yes | | HS Code | 29339990 |
| Indoline Usage And Synthesis |
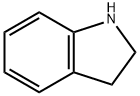
| Chemical Properties | Clear colorless to slightly brown liquid | | Uses | As an indole derivative, Indoline can be used in the preparation of various medicinal compounds such as potential α1-adrenoceptor (α1-AR) antagonists.
| | Uses | Indole is used in perfumery and in preparing tryptophan, one of the 20 amino acids commonly found in animal proteins. It has important application in the industry of plant growth. It is used to prepare indoleacetic acid (auxin) and other plant growth substances which help the development of roots in plant. Indole and its derivatives are widely used in making perfumes, dyes, agrochemicals and medicines. | | Uses | Reactant for preparation of:- Inhibitors of NOD1-Induced Nuclear Factor-κB Activation
- Sphingosine-1-phosphate 4(S1P4) receptor antagonists
- Cytotoxic cell cycle inhibitors
- 2-Aminopyridines
- PET agent for imaging of protein kinase C (PKC)
- Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes
- α4β2-Nicotinic acetylcholine receptor-selective partial agonists
- mGlu4 positive allosteric modulators
- Bacterial biofilm inhibitors
- Serotonin 5-HT6 receptor antagonists
| | Synthesis Reference(s) | Journal of the American Chemical Society, 96, p. 7812, 1974 DOI: 10.1021/ja00832a035Synthetic Communications, 13, p. 489, 1983 DOI: 10.1080/00397918308081827 |
| Indoline Preparation Products And Raw materials |
|