Other grades of this product :
| Ammonium oxalate Basic information |
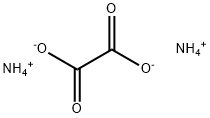
| Product Name: | Ammonium oxalate | | Synonyms: | diammoniumethanedioate;NA 2449;AMMoniuM oxalate, 95+%;oxalated’ammonium;AMMONIUM OXALATE TS;ETHANEDIOIC ACID DIAMMONIUM SALT;diammonium oxalate;OXALIC ACID DIAMMONIUM | | CAS: | 1113-38-8 | | MF: | C2H2O4.2H3N | | MW: | 124.1 | | EINECS: | 214-202-3 | | Product Categories: | | Mol File: | 1113-38-8.mol |
| Ammonium oxalate Chemical Properties |
| Melting point | 222-224°C | | Boiling point | 230.85°C (rough estimate) | | density | 1.5000 | | vapor pressure | 0Pa at 20℃ | | refractive index | 1.4350 (estimate) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Water (Slightly, Sonicated) | | form | Solid | | color | Colorless crystals | | Water Solubility | Soluble | | Stability: | Hygroscopic | | CAS DataBase Reference | 1113-38-8(CAS DataBase Reference) | | NIST Chemistry Reference | Ammonium oxalate(1113-38-8) | | EPA Substance Registry System | Diammonium oxalate (1113-38-8) |
| Ammonium oxalate Usage And Synthesis |
| Description | Ammonium oxalate, C2H8N2O4 (some times written as (NH4)2C2O4), is an oxalate salt with ammonium (sometimes as a monohydrate). It is a constituent of some types of kidney stone. Found also in guano. | | Chemical Properties | Ammonium oxalate is an odorless, colorless
crystalline material or powder. | | Uses | Ammonium oxalate is used as an analytical reagent and general reducing agent. It is commonly employed in soil chemical analysis to extract iron and aluminum from poorly-crystal line minerals. | | Definition | ChEBI: An ammonium salt consisting of ammonium and oxalate ions in a 2:1 ratio. | | Flammability and Explosibility | Notclassified | | Safety Profile | A poison. Can react
violently with (NaOCl+ ammonium
acetate). When heated to decomposition it
SYNS: ETHANEDIOIC ACID DIAMMONIUN SALT 0
OXALIC ACID, DIAIMMONIUM SALT
can emit toxic fumes of NH3 and NOx. See
also OXALATES. | | Potential Exposure | It is used in chemical analysis and to
make blueprint paper, explosives; a rust-removal ingredient
in metal polishes. | | Shipping | UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. UN1759 Corrosive solids, n.o.s., Hazard
class: 8; Labels: 8-Corrosive material, Technical Name
Required. | | Incompatibilities | Ammonium oxalate is a reducing agent
and also reacts as a base to neutralize acids and reacts with
oxidizers generating carbon dioxide. Incompatible with oxidizers (chlorates, hypochlorite solutions, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. |
| Ammonium oxalate Preparation Products And Raw materials |
|