Other grades of this product :
| (R)-(+)-Glycidol Basic information |
| (R)-(+)-Glycidol Chemical Properties |
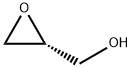
| Boiling point | 56-57 °C11 mm Hg(lit.) | | density | 1.116 g/mL at 20 °C(lit.) | | refractive index | n20/D 1.43(lit.) | | Fp | 178 °F | | storage temp. | -20°C | | Water Solubility | Completely miscible in water | | pka | 14.62±0.10(Predicted) | | form | neat | | color | Colorless to Light yellow | | optical activity | [α]23/D +15°, neat | | BRN | 79782 | | InChIKey | CTKINSOISVBQLD-GSVOUGTGSA-N | | CAS DataBase Reference | 57044-25-4(CAS DataBase Reference) | | NIST Chemistry Reference | Oxiranemethanol, (R)-(57044-25-4) |
| (R)-(+)-Glycidol Usage And Synthesis |
| Chemical Properties | Colorless to light yellow liqui | | Uses | (R)-(+)-Glycidol may be used in the following synthesis:- heteroarylpropane-2,3-diols, useful for combinatorial oligomer synthesis
- enantiomerically pure (2S,3S)- and (2S,3R)-threoninol and -hydroxyphenylalaninol
- 2-amino-1,4-anhydro-pentitol and 3-amino-1,5-anhydro-4-deoxy-hexitol with the arabino configuration
- (S)-4-tosyloxymethyl-2-oxazolidinone, required for the synthesis of protected amino alcohol derivatives
- 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine [(S)-HPMPC]
- chiral building block used to construct an epoxyvinyl iodide intermediate in a synthesis of a furanocembrane, a marine natural product
| | Uses | (R)-(+)-Glycidol can be used to construct chiral building blocks of epoxyvinyl iodide intermediates in the synthesis of furanocembrane, a marine natural product. | | General Description | Enantioselective esterification of (R)-(+)-glycidol with n-butyric acid in organic media using hybrid gel-entrapped lipase on Celite has been reported. | | Purification Methods | [S(-)-isomer, § also available on polymer support, has b 49-50o/7mm, 66-67o/19mm, [ ] D -1 5o(neat)], [R(+)-isomer has b 56 -5 6 . 5o/11mm, d 4 1.117, n D 1.429, [ ] D +15o (neat)]. Purify glycidol by fractional distillation. |
| (R)-(+)-Glycidol Preparation Products And Raw materials |
|