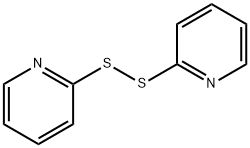
Other grades of this product :
| 2,2'-Dithiodipyridine Basic information |
| 2,2'-Dithiodipyridine Chemical Properties |
| Melting point | 56-58 °C(lit.) | | Boiling point | 356.1±17.0 °C(Predicted) | | density | 1.3078 (rough estimate) | | refractive index | 1.5500 (estimate) | | Fp | 210 °C | | storage temp. | 2-8°C | | solubility | methanol: soluble50mg/mL, clear to very slightly hazy, colorless to faintly brownish-yellow | | form | powder | | pka | 1.52±0.19(Predicted) | | color | Clear colorless to yellow to tan | | Water Solubility | 5 g/L (20 ºC) | | BRN | 154629 | | InChIKey | HAXFWIACAGNFHA-UHFFFAOYSA-N | | CAS DataBase Reference | 2127-03-9(CAS DataBase Reference) | | NIST Chemistry Reference | Pyridine, 2,2'-dithiobis-(2127-03-9) | | EPA Substance Registry System | Pyridine, 2,2'-dithiobis- (2127-03-9) |
| 2,2'-Dithiodipyridine Usage And Synthesis |
| Chemical Properties | white to yellowish crystalline powder | | Uses | 2,2'-Dipyridyldisulfide, sometimes known as DPS, is used for preparing thiols and activating or protecting carboxylic acid with triphenylphosphine. | | Uses | 2,2'-Dipyridyl disulfide is a useful reagent for determination of sulfhydryl groups, preparation of amino acid active esters and the thio esters of phosphoric acid. It acts as a peptide coupling reagent and as an oxidizing agent. It is also used for the activation of glycosides. | | Uses | Aldrithiol?-2 was used in the synthesis of adenosine-5′-phosphoimidazolide. It was employed with PPh3 as a condensing agent in the amidation of carboxylic acid enantiomers for HPLC separation. | | General Description | Aldrithiol?-2 is a nucleocapsid zinc finger targeting compound and can abrogate the infectivity of human immunodeficiency virus type-1virions. It is a thiol reagent. | | Biochem/physiol Actions | 2,2′-Dithiodipyridine mediates inter-unit disulfide cross-linking. It interacts with carbon nucleophiles to yield thiopyridyl derivatives. | | Purification Methods | Recrystallise the disulfide H2O from *C6H6/pet ether (6:7), ligroin or *C6H6. The picrate has m 119o (from EtOH). [Walter et al. Justus Liebigs Ann Chem 695 7785 1966, Marckwald et al. Chem Ber 33 1556 1900, Brocklehurst & Little Biochem J 133 67,78 1973, Beilstein 21 III/IV 48.] It has been used as a 1mM solution in EtOH for the spectrophotometric estimation of thiols. Essentially the thiol displaces half the disulfide molecule liberating the 2-mercaptopyridine anion, thereby shifting the from 340nm (of the disulfide) to 268nm (of the anion) at pH 9, or 278nm in H2O. (Compare |
| 2,2'-Dithiodipyridine Preparation Products And Raw materials |
|