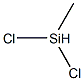
| Description | Methyl dichlorosilane, is a colorless liquid with a sharp irritating odor. It is a dangerous fire risk, corrosive, and water reactive. The flammable range is wide, from 6% on the lower end to 55% on the upper end. Boiling point is 107°F (41°C), flash point is 15°F (?9°C), and ignition temperature is more than 600°F (315°C). Specific gravity is 1.11, which is heavier than water. Vapors are heavier than air and will travel to ignition sources. It is immiscible in water and decomposes on contact to release hydrogen chloride gas. Methyl dichlorosilane is toxic by inhalation and skin absorption; it is irritating to the skin, eyes, and respiratory system. Contact with the material may cause burns to the eyes and skin. The four-digit UN identification number is 1242. The NFPA 704 designation is health 3, flammability 3, and reactivity 2. The white space at the bottom of the diamond has a W with a slash through it, indicating water reactivity. The primary use is in the manufacture of siloxanes, which are straight-chained compounds similar to paraffin hydrocarbons. |
| Chemical Properties | Methyl dichlorosilane is a clear, straw-colored
liquid. |
| Uses | Dichloromethylsilane is a monomer ('building block') in the production of silicone polymers. An intermediate (starting material) in the production of other organic and inorganic chemicals. In the electronics industry for the production of ultra-pure polysilicon and in the manufacture of semiconductors and photovoltaics. |
| Application | Provides better diastereoselective reductive aldol reactionbetween an aldehyde and an acrylate ester than othersilanes. Forms high-boiling polymeric by-products uponaqueous work-up. |
| General Description | A colorless fuming liquid with a pungent odor. Flash point: -26°F; Boiling point: 41°C (106°F) . Vapors heavier than air. Vapor and liquid may cause burns. Denser than water and decomposed by water to form hydrochloric acid, a corrosive material. |
| Reactivity Profile | Chlorosilanes, such as Dichloromethylsilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases. Impact of dichlorosilane causes mixtures to burst into flame. This is the case, when the dichlorosilane and most likely related substances are mixed with oxidants such as potassium permanganate, lead oxide, copper oxide, or silver oxide, even under inert gas atmosphere [Bretherick, 1995, pg. 193]. With Dichloromethylsilane, toxic hydrogen chloride and phosgene gases may be formed when burned. |
| Hazard | Flammable, dangerous fire risk. Very toxic. |
| Health Hazard | Inhalation causes irritation of respiratory tract; heavy exposure can cause pulmonary edema. Contact of liquid with skin or eyes causes severe burns. Ingestion causes burns of mouth and stomach. |
| Chemical Reactivity | Reactivity with Water Reacts violently to form hydrogen chloride (hydrochloric acid); Reactivity with Common Materials: Reacts with surface moisture to evolve hydrogen chloride, which is corrosive to common metals; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flood with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
| Safety Profile | Moderately toxic by
inhalation. Corrosive. A severe irritant to
skin, eyes, and mucous membranes. Ignites
spontaneously in air. A very dangerous fire
hazard when exposed to heat or flame.
Forms impact-sensitive explosive mixtures
with potassium permanganate, lead(Ⅱ)
oxide, lead(Ⅳ) oxide, copper oxide, silver
oxide. To fight fire, use water, foam, CO2,
mist. When heated to decomposition it
emits toxic fumes of Cl-. See also
CHLOROSILANE. |
| Potential Exposure | This material is used to make
siloxanes and other silicone polymer (polysiloxane) materials.
Incompatibilities: May form explosive mixture with air.
Reacts violently with water producing heat, corrosive
hydrochloric acid and flammable hydrogen. Methyl dichlorosilane may spontaneously ignite on contact with air (even
under inert gas) and on contact with potassium permanganate, lead(II) oxide; copper oxide; silver oxide. Violent
reaction with oxidizers. Decomposes on contact with hot
surfaces or flames producing toxic and corrosive fumes
including silicon oxides, hydrogen chloride, and phosgene.
Decomposes on contact with alkaline compounds producing
highly flammable hydrogen gas. Corrodes many metals in
presence of water. Attacks some plastics, rubber and
coatings. |
| Shipping | UN1242 Methyldichlorosilane Hazard Class:
4.3; Labels: 4.3-Dangerous when wet material; 8-Corrosive
material, 3-Flammable liquid. |
| Purification Methods | Impurities are generally other chloromethyl silanes. Distil it through a conventional Stedman column (p 11) of 20 theoretical plates or more. It should be protected from H2O by storing over P2O5. [Stck & Somieski Chem Ber 52 695 1919, Sauer J Am Chem Soc 68 962 1946, Beilstein 4 IV 4096.] |
| Waste Disposal | See “Spill Handling.” |