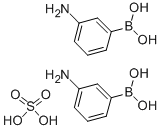
Other grades of this product :
| 3-Aminobenzeneboronic acid hemisulfate salt Basic information |
| 3-Aminobenzeneboronic acid hemisulfate salt Chemical Properties |
| Melting point | ≥300 °C | | storage temp. | Inert atmosphere,2-8°C | | Water Solubility | Soluble in water | | form | Crystalline Powder | | color | White to slightly beige | | Sensitive | Hygroscopic | | BRN | 8094151 | | InChIKey | XMVGYYBQCXVVHU-UHFFFAOYSA-N | | CAS DataBase Reference | 66472-86-4 |
| 3-Aminobenzeneboronic acid hemisulfate salt Usage And Synthesis |
| Chemical Properties | White to slightly beige crystalline powder | | Uses | 3-Aminobenzeneboronic acid hemisulfate salt is a boronic acid derivative, which can be used as an intermediate in pharmaceutical synthesis. Adsorbent additive for the chromatographic separation of 3'-terminal polynucleotides from RNA. | | Application | 3-Aminophenylboronic acid hemisulfate salt is used in the synthesis of:Phenylboronic acid-functionalized inverse opal hydrogels within microfluidic flow cells for glucose sensing.Phenylboronic acid-salicylhydroxamic acid-derived complexing reagent for protein immobilization on chromatographic support.It can also be used in the preparation of phenylboronic acid (PBA) monolayer-modified Au electrode for the voltammetric sensing of uridine and cytidine. | | Preparation | 3-Aminobenzeneboronic acid hemisulfate salt synthesis: Benzenamine, 3-bromo-N-(diphenylmethylene)- (80,0 g; 0.24 moles) was added to the cryogenic reactor along with 1.4 equivalents of triisopropyl borate and dissolved in tetrahydrofuran. The resulting solution was cooled to -80°C under an inert atmosphere and 1.35 equivalents of n-butyllithium (as a solution in n-hexane) was added maintaining the internal temperature. When complete conversion was achieved, the reaction mixture was added to a dilute mixture of sulfuric acid (1 M) and toluene. The layers were separated to obtain an aqueous solution of 3-aminophenylboronic acid hydrogen sulfate (assay yield: 85%). 3-Aminobenzeneboronic acid hemisulfate salt can be obtained by precipitating a 0.5 M aqueous solution at pH 7.2 (pH corrected with 33% aqueous NaOH) at a temperature of 0-5°C. Filtration and drying under vacuum at 40°C for 8 hours yielded 21.7 g of 3-Aminobenzeneboronic acid hemisulfate salt (0.16 moles; 66% molar yield). |
| 3-Aminobenzeneboronic acid hemisulfate salt Preparation Products And Raw materials |
|