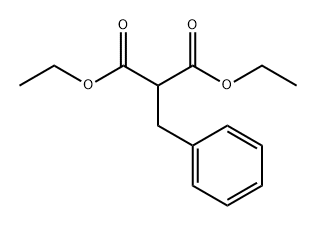
| Diethyl benzylmalonate Basic information |
| Product Name: | Diethyl benzylmalonate |
| Synonyms: | BENZYLMALONIC ACID DIETHYL ESTER;DIETHYL BENZYLMALONATE;AKOS BBS-00006590;(phenylmethyl)-propanedioicacidiethylester;Diethyl 2-benzylmalonate;Malonic acid, 2-benzyl-, diethyl ester;Malonic acid, benzyl-, diethyl ester;1,1-Bis(ethoxycarbonyl)-2-phenylethane |
| CAS: | 607-81-8 |
| MF: | C14H18O4 |
| MW: | 250.29 |
| EINECS: | 210-142-7 |
| Product Categories: | Building Blocks;C12 to C63;Pharmaceutical Intermediates;Carbonyl Compounds;Chemical Synthesis;Organic Building Blocks;Aromatic Esters;TheMalonateRamificationProducts;Aromatics Compounds;Aromatics;C12 to C63;Carbonyl Compounds;Esters;bc0001 |
| Mol File: | 607-81-8.mol |
| Diethyl benzylmalonate Chemical Properties |
| Boiling point | 162-163 °C/10 mmHg (lit.) |
| density | 1.064 g/mL at 25 °C (lit.) |
| vapor pressure | 0.26Pa at 25℃ |
| refractive index | n |
| Fp | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform, Methanol |
| pka | 12.56±0.46(Predicted) |
| form | Colourless Liquid |
| color | Colorless to Almost colorless |
| Water Solubility | immiscible |
| BRN | 615793 |
| InChIKey | ICZLTZWATFXDLP-UHFFFAOYSA-N |
| LogP | 2.76 |
| CAS DataBase Reference | 607-81-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanedioic acid, (phenylmethyl)-, diethyl ester(607-81-8) |
| EPA Substance Registry System | Propanedioic acid, 2-(phenylmethyl)-, 1,3-diethyl ester (607-81-8) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 24/25-36-26 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29173980 |
| MSDS Information |
| Provider | Language |
|---|---|
| Diethyl benzylmalonate | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Diethyl benzylmalonate Usage And Synthesis |
| Chemical Properties | Colourless Liquid |
| Uses | Diethyl Benzylmalonate (cas# 607-81-8) is a compound useful in organic synthesis. |
| Uses | Diethyl benzylmalonate was used in the synthesis of macrocyclic ligands and determination of stability constants of their Cu(II), Ni(II) and Co(II) complexes. It was used as starting reagent for the synthesis of 2-benzyl-1,3-propane diol. |
| Synthesis Reference(s) | The Journal of Organic Chemistry, 31, p. 620, 1966 DOI: 10.1021/jo01340a523 |
| General Description | Diethyl benzylmalonate on condensation with 2-amino-benzimidazole yields 3-benzyl-4-hydroxypyrimido[1,2-a]benzimidazole-2-one. |
| Diethyl benzylmalonate Preparation Products And Raw materials |
Please leave a message for us or use the following ways to contact us, we will reply to you as soon as possible, and provide you with the most sincere service, thank you.