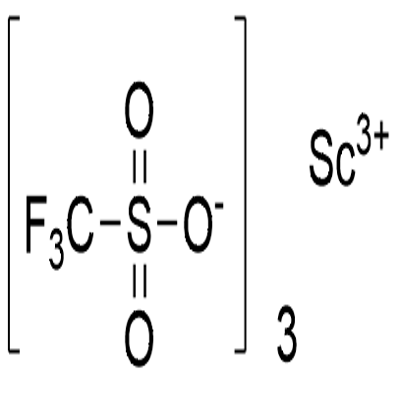
| Description | Scandium trifluoromethanesulfonate, commonly called Scandium(III) triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3? anions.Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates. |
| Chemical Properties | White powder |
| Uses | Scandium(III) trifluoromethanesulfonate is widely used as a catalyst in hydrothiolation, selective two-electron reduction of oxygen by ferrocene derivatives and vinylogous Fridel-crafts alkylation of indoles and pyrrole in water. It is involved in the Mukaiyama aldol addition and stereochemically catalyzes the radical polymerization of acrylates. It acts as a Lewis acid catalyst and used in the synthesis of bullvalone via a stabilized sulfur ylide. |
| Uses | Scandium Triflate is an important catalyst used in Friedel-Crafts acylation, Baylis-Hillman reaction and other carbon-carbon bond forming reactions. |
| Application | Scandium(III) triflate was used as a catalyst in:Hydrothiolation reaction of aromatic and aliphatic thiols.Selective two-electron reduction of O2 by ferrocene derivatives.Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.Synthesis of β-cyanoketones.Combination with triethylsilane to reductively open functionalized pyranoside rings.The key steps of synthesis of bullvalone via a stabilized sulfur ylide. |
| Reactions | Water tolerant Lewis acid. Commonly used in a range of Lewis acid catalyzed reactions. Efficient metal source for Lewis acid catalyzed asymmetric reactions. Catalyzes Friedel-Crafts alkylation, acylation and related reactions. Catalyzes various domino- and multi-component processes. Catalyzes electrophilic additions of alpha-diazoesters with ketones. Catalyzes carbon insertion reactions.
|
| General Description | Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates. |