Other grades of this product :
| Desvenlafaxine succinate Basic information |
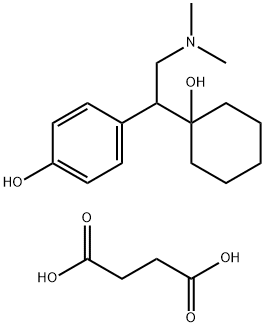
| Product Name: | Desvenlafaxine succinate | | Synonyms: | Desvenlafaxine succinate;4-(2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl)phenol succinate hydrate;4-(2-(Dimethylamino)-1-(1-hydroxycyclohexyl)ethyl)phenol succinate monohydrate;2-(1-Hydroxycyclohexyl)-2-(4-hydroxyphenyl)ethyl]dimethylammonium3-carboxypropanoatemonohydrate;Desvenlafaxine succinate monohydrate;Pristiq;Unii-zb22enf0xr;Desvenlafaxine (succinate hydrate) | | CAS: | 386750-22-7 | | MF: | C20H31NO6 | | MW: | 381.47 | | EINECS: | | Product Categories: | Inhibitors;APIs | | Mol File: | 386750-22-7.mol |
| Desvenlafaxine succinate Chemical Properties |
| storage temp. | Store at +4°C |
| Desvenlafaxine succinate Usage And Synthesis |
| Description | Desvenlafaxine Succinate is a dual serotonin and norepinephrine
reuptake inhibitor (SNRI) that was approved for the
treatment of major depressive disorder (MDD) in the United
States in 2008. In order to improve the efficacy and
safety profile of venlafaxine, Wyeth discovered and developed
one of the major metabolites of venlafaxine, namely the
O-desmethyl metabolite (desvenlafaxine). Desvenlafaxine is
also being developed for the treatment of moderate to severe
vasomotor symptoms associated with menopause (i.e., hot
flashes and night sweats) and is also in phase III clinical trials
to study it’s effectiveness in treating fibromyalgia and
neuropathic pain. | | Uses | Antianxiety therapeutic | | Synthesis | Desvenlafaxine has been prepared via
two different routes, and both are described in the scheme.
The first route involved simple demethylation of venlafaxine
(67) using L-selectride in dimethoxyethane giving desvenlafaxine
68 as its free base in 91% yield. Compound
68 was then recrystallized with succinic acid in acetone/
water to give desvenlafaxine succinate (VIII) in 86%
yield. An alternative method to prepare desvenlafaxine is
described in the bottom portion of the scheme. 4-Benzyloxyphenylacetic
acid 69 was converted to its corresponding
acid chloride upon treatment with thionyl chloride and catalytic
DMF in refluxing methylene chloride. The crude
reaction mixture was added to a solution of dimethylamine
hydrochloride and triethyl amine in methylene chloride at 5
°C to give dimethylacetamide 70 in 90% yield. Deprotonation
of 70 with LHMDS in THF at -70 °C followed by addition
of cyclohexanone gave alcohol 71 in 82% yield. Reduction
of the acetamide with borane THF complex in refluxing THF produced dimethyl amine 72 in 66% yield. Catalytic
hydrogenation in the presence of 20% Pd/C effected debenzylation
of 72 to give desvenlafaxine free base 68 in 87%
yield. |
| Desvenlafaxine succinate Preparation Products And Raw materials |
|