Other grades of this product :
| CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)RUTHENIUM (II) Basic information |
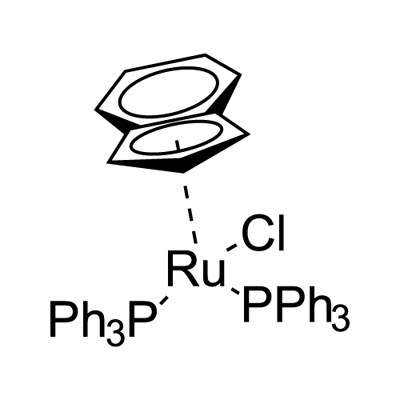
| Product Name: | CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)RUTHENIUM (II) | | Synonyms: | C9H7RuCl2(C18H15P);Reaxys ID: 11961247;CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)R&;Chloro(indenyl)bis(triphenylphosphine)ruthenium(II),dichloromethaneadduct,min.98%;Chloro(indenyl)bis(triphenylphosphine)rutheniuM(II),dichloroMethane adduct,98%;Ruthenium,chloro[(1,2,3,3a,7a-h)-1H-inden-1-yl]bis(triphenylphosphine)-;Chloro(indenyl)bis(triphenylphosphine)ruthenium(II) may contain <=1 molar equivalent dichloromethane/acetone;CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)RUTHENIUM(II),DICHLOROMETHANEADDUCT,MIN. | | CAS: | 99897-61-7 | | MF: | C45H37ClP2Ru5* | | MW: | 776.25 | | EINECS: | | Product Categories: | Ru;organometallic complexes | | Mol File: | 99897-61-7.mol |
| CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)RUTHENIUM (II) Chemical Properties |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | form | crystal | | color | red-brown |
| CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)RUTHENIUM (II) Usage And Synthesis |
| Uses | Catalyst for:- Single-chain folding of polymers
- Domino redox bicycloisomerization
- Selective radical addition with a designed heterobifunctional halide
- Tandem living radical polymerization via in situ monomer transformation with alcohols
- Atom-economical synthesis of nitrogen heterocycles
Reactant for the synthesis of chiral-at-metal ruthenium allenylidene complexes |
| CHLORO(INDENYL)BIS(TRIPHENYLPHOSPHINE)RUTHENIUM (II) Preparation Products And Raw materials |
|