Other grades of this product :
| 2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Basic information |
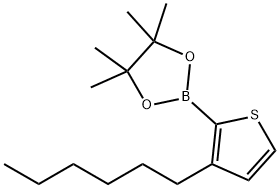
| Product Name: | 2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane | | Synonyms: | 2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
3-Hexyl-2-thiopheneboronic Acid Pinacol Ester;3-Hexyl-2-thiopheneboronic acid pinacol ester;2-(3-Hexylthiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;3-Hexylthiophene-2-boronic acid pinacol ester 95%;3-hydroxy-2,3-dimethylbutan-2-yl hydrogen (3-hexylthiophen-2-yl)boronate;2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;3-Hexylthiophene-2-boronic acid pinacol ester;3-Hexyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene | | CAS: | 850881-09-3 | | MF: | C16H27BO2S | | MW: | 294.26 | | EINECS: | | Product Categories: | Boronate Esters;Boronic Acids and Derivatives;Chemical Synthesis;Heteroaryl Boronate Esters;Materials Science;Organic and Printed Electronics;Organometallic Reagents;Synthetic Tools and Reagents;Thiophene Monomers and Building Blocks | | Mol File: | 850881-09-3.mol |
| 2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Chemical Properties |
| Boiling point | 385.3±30.0 °C(Predicted) | | density | 0.983 g/mL at 25 °C | | refractive index | n20/D 1.492 | | Fp | 110 °C | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | form | clear liquid | | color | Colorless to Light yellow |
| WGK Germany | 3 | | HS Code | 2934999090 |
| 2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Usage And Synthesis |
| Uses | Reagent used for• ;Suzuki-Miyaura cross-coupling reactions1 • ;p-type/n-type switching of ambipolar bithiazole-benzothiadiazole-based polymers in solar cells1 • ;Hierarchical self-assembly of semiconductor functionalized peptide a-helixes and optoelectronic properties2 Reagent used in Preparation of• ;Photovoltaic materials, polymers, and thiophene-based compounds with photophysical, electrochemical, and fluorescent properties3,4,5• ;Polymer solar cells for Low band gap poly(1,4-arylene-2,5-thienylene)s with benzothiadiazole units6 • ;Dithienothiophene-based dyes | | Uses | Reagent used for- Suzuki-Miyaura cross-coupling reactions
- p-type/n-type switching of ambipolar bithiazole-benzothiadiazole-based polymers in solar cells
- Hierarchical self-assembly of semiconductor functionalized peptide a-helixes and optoelectronic properties
Reagent used in Preparation of- Photovoltaic materials, polymers, and thiophene-based compounds with photophysical, electrochemical, and fluorescent properties
- Polymer solar cells for Low band gap poly(1,4-arylene-2,5-thienylene)s with benzothiadiazole units
- Dithienothiophene-based dyes for dye-sensitized solar cells
|
| 2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Preparation Products And Raw materials |
|