Other grades of this product :
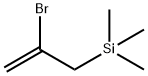
| (2-BROMOALLYL)TRIMETHYLSILANE Basic information |
| (2-BROMOALLYL)TRIMETHYLSILANE Chemical Properties |
| Boiling point | 82-85 °C/60 mmHg (lit.) | | density | 1.121 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.462(lit.) | | Fp | 87 °F | | storage temp. | 2-8°C | | solubility | sol alcohol, acetone, ether, THF, pentane; insol water. | | BRN | 3600686 |
| Hazard Codes | Xi | | Risk Statements | 10-36/37/38 | | Safety Statements | 16-26-36 | | RIDADR | UN 1993 3/PG 3 | | WGK Germany | 3 | | F | 8-10 | | HazardClass | 3.2 | | PackingGroup | III |
| (2-BROMOALLYL)TRIMETHYLSILANE Usage And Synthesis |
| Physical properties | bp 46–50°C/20 mmHg,64–65°C/38–
39 mmHg. | | Uses | 2-Bromo-3-trimethylsilyl-1-propene can be used as synthon for CH2=C?CH2TMS1–3 and CH2=CBrC?H2;2 for
synthesis of 1-trimethylsilylmethyl-substituted 1,3-butadienes.The 1-trimethylsilylmethylvinyl
anion CH2=C(M)CH2TMS (2) (M = Li, Mg, Cu,
etc.), readily prepared from 2-bromo-3-trimethylsilyl-1-propene
(1) under typical conditions, allows the introduction of the
synthetically useful 1-trimethylsilylmethylvinyl group to a wide
variety of substrates. Ring opening of 1-butene oxide with the
Grignard reagent (2) (M = MgBr) in the presence of copper(I)
iodide gives only one regioisomer. Subsequent desilylative oxidation
of this allyl alcohol to α-methylene-γ-lactones provides
further utility of (1) as a 1-hydroxymethylvinyl anion equivalent,
i.e. CH2=?C?CH2OH (eq 1).Alternatively, the alcohol
from trans-2,3-epoxybutane provides a route to the unstable sixmembered
β,γ-unsaturated lactone (eq 2).The copper-catalyzed
1,4-addition to the typically unreactive mesityl oxide proceeds
smoothly. The versatility of the allylsilane moiety is again illustrated
in the ethylaluminum dichloride-induced cyclization of the
adduct to a tertiary cyclopentanol in high yield (eq 3). | | Preparation | reaction of 2,3-dibromopropene
with lithium (trimethylsilyl)cuprate in HMPA at 0°C
(63–90%);(2) reaction of 2,3-dibromopropene with trichlorosilane
in the presence of trichlorosilane and copper(
I) chloride, followed by treatment with methylmagnesium
bromide (63–71%). | | Purification Methods | It is fractionally distilled through an efficient column. It is flammable. [Trost & Chan J Am Chem Soc 104 3733 1982, Trost & Coppola J Am Chem Soc 104 6879 1982.] |
| (2-BROMOALLYL)TRIMETHYLSILANE Preparation Products And Raw materials |
|