Other grades of this product :
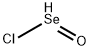
| Product Name: | Selenium Oxychloride | | Synonyms: | Seleniumdichlorideoxide,97%;Selenyl chloride;Selenium oxidichloride;Selenium(IV) dichlorideoxide;Selenium(IV) oxychloride,97%;Selenium(IV) oxychlorideSeleninyl chloride;SELENIUM DICHLORIDE OXIDE 97%;Seleniumdichlorideoxide,99%(metalsbasis) | | CAS: | 7791-23-3 | | MF: | Cl2OSe | | MW: | 165.87 | | EINECS: | 232-244-0 | | Product Categories: | metal oxyhalide;Inorganics | | Mol File: | 7791-23-3.mol |
| Selenium Oxychloride Chemical Properties |
| Melting point | 9 °C | | Boiling point | 180 °C(lit.) | | density | 2.43 g/mL at 25 °C(lit.) | | refractive index | 1.651 | | Fp | 176.4°C | | solubility | Miscible with chloroform, carbon disulphide and benzene. | | form | Liquid | | color | Yellow | | Specific Gravity | 2.42 | | Water Solubility | decomposed in H2O to HCl, selenious acid; miscible with CCl4, CHCl3, CS2, benzene, toluene [MER06] | | Sensitive | Moisture Sensitive | | Merck | 14,8435 | | Exposure limits | ACGIH: TWA 0.2 mg/m3NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 | | Stability: | Stable, but reacts with water and moisture. Incompatible with water, potassium, phosphorus. | | CAS DataBase Reference | 7791-23-3(CAS DataBase Reference) | | EPA Substance Registry System | Selenium oxychloride (7791-23-3) |
| Selenium Oxychloride Usage And Synthesis |
| Chemical Properties | Selenium Oxychloride is a colorless to yellow, fuming liquid. Insoluble in water and denser than water. Selenium Oxychloride is toxic and extremely vesicant. When 0.01 cc is applied to the skin of rabbits,death occurs in less than 24 hours. It readily destroys skin on contact, causing third-degree burns unless immediately removed with water.
The first record of the use of selenium oxychloride in organic reactions dates back to the year 1879, when Friedrich Clausnizer1 obtained acetic anhydride by treating acetic acid with this reagent.
Selenium oxychloride may be used as a reagent for the preparation of α-chloro ketones.Selenium oxychloride is miscible in CCl4, carbon disulfide and benzol.
It is used as a solvent, chlorinating agent and resin plasticizer. | | Uses | Selenium oxychloride is a solvent for synthetic phenolic resins and many other substances.
| | Preparation | Selenium oxychloride may be prepared by several methods: (1) by passing chlorine gas into a suspension of selenium dioxide in carbon tetrachloride, (2) by heating thionyl chloride, SOCl2, with selenium dioxide, (3) by dehydration of dichloroselenious acid, H2Se(Cl2)O2, and (4) by fusion of selenium dioxide, selenium, and calcium chloride.
| | Chemical Properties | Selenium oxychloride is a colorless to yellowish liquid. Fumes in air. | | Physical properties | Pale yellow or colorless liquid; corrosive; refractive index 1.651 at 20°C; density 2.42 g/mL at 22°C; freezes at 8.5°C; boils at 176.4°C; decomposes at 176.4°C; decomposes in water forming hydrochloric acid and selenious acid; soluble in carbon disulfide, carbon tetrachloride, chloroform, benzene, and toluene. | | Uses | Selenium(IV) chloride oxide is used as a polar solvent for synthetic phenolic resins. It is also used to prepare selenium oxybromide and selenium nitride. It acts as a chlorinating agent. | | Uses | selenium oxychloride is used as solvent. | | Production Methods | Selenium oxychloride (SeOCl2) is a powerful solvent, chlorinating
agent, and resin plasticizer used in the chemical
industry. | | General Description | A colorless to light-colored liquid. Insoluble in water and denser than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals. | | Air & Water Reactions | Fumes in air. Insoluble in water and denser than water. Decomposed in water or moist air to form hydrochloric acid and selenious acid [Merck 11th ed. 1989]. | | Reactivity Profile | Red phosphorus reacts in the cold with SELENIUM OXYCHLORIDE evolving light and heat; white phosphorus reacts explosively [Mellor 10:906 1946-47]. When potassium is brought into contact with SELENIUM OXYCHLORIDE in the cold, an explosion occurs [Mellor 10:908 1946-47]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Powdered antimony ignites on contact with the chloride, [Mellor, 1947, Vol. 10, 906]. With metal oxides, i.e. silver oxide, light is evolved and heat sufficient to decompose the mixture, [Mellor, 1947, Vol. 10, 909]. | | Hazard | Extremely hazardous, toxic irritant, poison,
severe vesicant.
| | Health Hazard | SELENIUM OXYCHLORIDE is very toxic and may cause death or permanent injury after very short exposures to small quantities. Inhalation of small quantities may be corrosive and irritating to the respiratory tract. It can burn and irritate the skin and eyes and cause burns when ingested. Long-term exposure to selenium compounds may be a cause of amyotrophic lateral sclerosis in humans. Populations at special risk include those with a history of dermatitis, chronic bronchitis, skin allergies, respiratory tract infections, liver or kidney disease, jaundice, or albuminuria. Women of child-bearing age are also considered at risk. | | Fire Hazard | When SELENIUM OXYCHLORIDE is heated to decomposition, or in contact with acids or acid fumes, highly toxic chloride and selenium fumes are evolved. Hydrochloric acid and selenious acid are produced by reaction with water. Decomposed by water. Reacts violently with powdered antimony, red and white phosphorus, disilver oxide, lead oxides, and potassium. Avoid water, moist air. | | Safety Profile | Poison by skin contact and subcutaneous routes. Human systemic effects by skin contact with very small amounts: primary irritant, corrosive. Explodes on contact with potassium, white phosphorus. Ignites on contact with antimony. Vigorous reaction with metal oxides (e.g., silver oxide, lead@) oxide, lead(Ⅳ) oxide, lead(II)(IV) oxide). When heated to decomposition it emits very toxic fumes of Cland Se. See also SELENIUM COMPOUNDS and CHLORIDES.
| | Potential Exposure | This material is used as a solvent for many substances, including metals and as a chlorinating agent; and resin plasticizer; as an ionizing solvent; for monochlorination of ketones. | | Shipping | UN2879 Selenium oxychloride, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous material. | | Incompatibilities | Water and air reactive releasing strong acids. The aqueous solution is a strong acid and oxidizer.Reacts violently with bases, reducing agents, hydrides, powdered antimony, red, and white phosphorus, disilver oxide, lead oxide; powdered metals, and potassium. Note: Never pour water into this substance; when dissolving or diluting always add it slowly to the water |
| Selenium Oxychloride Preparation Products And Raw materials |
|