Other grades of this product :
| Barium manganate Chemical Properties |
| density | 4.85 g/mL at 25 °C (lit.) | | form | Fine Crystalline Powder | | Specific Gravity | 4.85 | | color | Blue to dark blue-black | | Water Solubility | Slightly soluble in water | | Sensitive | Moisture Sensitive | | Merck | 14,981 | | Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3OSHA: Ceiling 5 mg/m3NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 | | EPA Substance Registry System | Manganic acid (H2MnO4), barium salt (1:1) (7787-35-1) |
| Hazard Codes | Xn,O | | Risk Statements | 20/22-8 | | Safety Statements | 28-28A | | RIDADR | UN 1479 5.1/PG 2 | | WGK Germany | 1 | | F | 21 | | TSCA | Yes | | HazardClass | 5.1 | | PackingGroup | III | | HS Code | 28416900 |
| Barium manganate Usage And Synthesis |
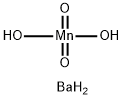
| Description | Barium manganate (chemical formula BaMnO4) is a type of manganates in which the manganese is in a +6 oxidation state. It is obtained by heating manganese dioxide with barium carbonate or nitrate, or by precipitating potassium manganate with barium chloride.1 Barium manganate gives a green pigment, which was used to make manganese blue. Barium manganate can be used in the oxidation of a variety of functional groups efficiently and selectively: alcohols to carbonyls, alcohols to aldehydes, diols to lactones, thiols to disulfides, aromatic amines to azo-compounds, hydroquinone to p-benzoquinone, benzylamine to benzaldehyde, etc. 2,3
| | References |
- N. Eastaugh, V. Walsh, T. Chaplin, R. Siddall, Pigment Compendium, 2008, ISBN: 978-0-7506-8980-9
- https://en.wikipedia.org/wiki/Barium_manganate
- http://reag.paperplane.io/00000118.htm
| | Chemical Properties | blue to dark blue-black fine crystalline powder | | Uses | As pigment in fresco painting instead of Scheele's green because not so poisonous as latter. As mild oxidizing agent in organic synthesis. | | Uses | Useful for the selective oxidation of diols. | | Purification Methods | Wash the salt with conductivity H2O by decantation until the supernatant gives a faint test for Ba2+ . Remove excess H2O in a vacuum (IMPORTANT), then heat at 100o and the last traces of H2O are removed in a vacuum desiccator over P2O5. Store it over KOH. It disproportionates in hot H2O or dilute acid into Ba(MnO2)2 and MnO2, and is a mild oxidant. [Schlezinger & Siems J Am Chem Soc 46 1965 1924, Nyholm & Woolliams Inorg Synth XI 56 1968.] |
| Barium manganate Preparation Products And Raw materials |
|