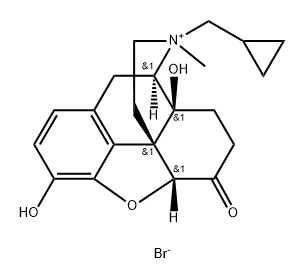
Other grades of this product :
| Naltrexone methylbromide Basic information |
| Naltrexone methylbromide Chemical Properties |
| Melting point | 237-239°C | | storage temp. | -20°C | | solubility | H2O: ≥5mg/mL | | form | powder | | color | white to beige |
| Hazard Codes | T,N | | Risk Statements | 25-50 | | Safety Statements | 45-61 | | RIDADR | UN 2811 6.1 / PGIII | | WGK Germany | 3 | | RTECS | QD0188550 | | HS Code | 2934990002 |
| Naltrexone methylbromide Usage And Synthesis |
| Description | The widespread efficacy of opioids in treating patients with moderate to
severe acute and chronic pain is often accompanied by untoward side
effects. In particular, opioid-induced bowel dysfunction is one of the
more common and debilitating consequences afflicting up to 50% of
patients. To counteract the peripherally-mediated adverse effects, opioid
antagonists such as naloxone, naltrexone, and nalmephene are sometimes prescribed. The latest market entry exploits a strategic
modification of naltrexone to lower its lipid solubility and increase its
polarity: quaternization of the amine of naltrexone by methylation
(methyl bromide) prevents crossing of the blood–brain barrier thereby
creating an effective peripheral antagonist. Despite a loss of potency
upon methylation, methylnaltrexone antagonizes opioid binding at
m-opioid receptors with an IC50 of 70 nM. Its affinity for k-opioid receptors
is approximately eightfold less (IC50= 575 nM) with no significant binding
to d-opioid, orphanin, or non-opioid receptors. Methylnaltrexone bromide
has been approved for the treatment of opioid-induced constipation in
patients with advanced illness receiving palliative care.Regarding metabolism, methylnaltrexone bromide is eliminated primarily as intact drug (85% based on administered radioactivity) by slightly more renal than hepatic clearance.The most common adverse events were abdominal pain and flatulence followed by nausea, dizziness, and diarrhea. | | Chemical Properties | Pale Pink Solid | | Originator | University of Chicago (United States) | | Uses | A metabolite of Naltrexone (N285750). Methylnaltrexone (MNTX), a selective μ-opioid receptor antagonist, functions as a peripherally acting receptor antagonist in tissues of the gastrointestinal tract. | | Uses | Methylnaltrexone bromide has been used as a drug to measure plasma protein binding (PPB), permeability (Pm) and the membrane coefficient (KIAM) for the prediction of blood brain barrier (BBB) penetration. It is also used as a mu-opioid receptor (MOR) antagonist to abrogate morphine tolerance and opioid-induced hyperalgesia (OIH). | | Brand name | Relistor | | General Description | Methylnaltrexone does not cross blood brain barrier and does not affect the opioid effects in the brain, such as analgesia. It is used to treat opioid-induced constipation (OIC). | | Biochem/physiol Actions | Methylnaltrexone bromide is a narcotic antagonist. It is a peripheral mu-opiod receptor antagonist that cannot cross the blood-brain barrier. It reverses many opioid side-effects without interfering with pain relief. | | Synthesis | The
synthesis of methylnaltrexone bromide proceeds in a
straightforward manner via the alkylation of naltrexone 90
in the following scheme. Naltrexone 90 was reacted with methyl
bromide in 1-methyl-2-pyrrolidinone at 60 °C. The resulting
crude product was treated with sodium methoxide in methanol/
water at 55 °C to remove any undesired phenolic (Oalkylated)
side-products. The resulting crude sodium salt was
treated with hydrobromic acid in methanol/water and upon
crystallization gave methylnaltrexone bromide (XII) in 35%
overall yield. |
| Naltrexone methylbromide Preparation Products And Raw materials |
|