Other grades of this product :
| SODIUM TRICHLOROACETATE Basic information |
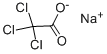
| Product Name: | SODIUM TRICHLOROACETATE | | Synonyms: | ERBITOX T95G;SODIUM TRICHLOROACETATE;TCA-SODIUM;TCA SODIUM SALT;TRICHLOROACETIC ACID SODIUM SALT;Aceticacid,trichloro-,sodiumsalt;sodio(tricloroacetatodi);sodium(trichloracetatede) | | CAS: | 650-51-1 | | MF: | C2Cl3NaO2 | | MW: | 185.37 | | EINECS: | 211-479-2 | | Product Categories: | | Mol File: | 650-51-1.mol |
| SODIUM TRICHLOROACETATE Chemical Properties |
| Melting point | 300 °C | | density | 0.9 g/cm3 | | storage temp. | Sealed in dry,Room Temperature | | form | Powder or Granular Powder | | pka | 0.6[at 20 ℃] | | color | White to off-white | | Water Solubility | Soluble in water, ethanol, methanol, acetone. | | Sensitive | Hygroscopic | | Merck | 14,9627 | | BRN | 3572664 | | InChIKey | SAQSTQBVENFSKT-UHFFFAOYSA-M | | LogP | -2.67 at 25℃ | | CAS DataBase Reference | 650-51-1(CAS DataBase Reference) | | EPA Substance Registry System | Sodium trichloroacetate (650-51-1) |
| Hazard Codes | Xi,N | | Risk Statements | 37-50/53 | | Safety Statements | 46-60-61 | | RIDADR | UN 3077 9/PG 3 | | WGK Germany | 2 | | RTECS | AJ9100000 | | TSCA | Yes | | HazardClass | 9 | | PackingGroup | III | | HS Code | 29154000 | | Hazardous Substances Data | 650-51-1(Hazardous Substances Data) | | Toxicity | LD50 orl-rat: 3320 mg/kg KSKZAN 18(3),46,80 |
| SODIUM TRICHLOROACETATE Usage And Synthesis |
| Chemical Properties | White to almost white powder | | Uses | As a decalcifier and fixative in microscopy; also as a precipitant of protein. As herbicide. | | Uses | Sodium trichloroacetate, is used in acid binding reagent in the textile industry in the dying process. Sodium trichloroacetate has been reported previously to be an effective denaturation reagent for proteins was applied to poly(l-lysine) and poly(l-glutamic acid) to see its effects on a coil-to-helix transition and on the chemical reactivities of the ε-amino group of poly(l-lysine). Addition of sodium trichloroacetate to poly-(l-lysine) induced a helical conformation even at neutral pH where the ε-amino group of the polymer was protonated. | | Uses | Sodium trichloroacetate with trichloroacetic acid (TCA) undergoes rapid decarboxylation in dimethylformamide (DMF) in the presence of aldehydes to form nucleophilic trichloromethyl anion, which then adds to the aldehydes to form trichloromethyl carbinols. It can also undergo thermal decarboxylation in aprotonic solvents in the presence of olefins to generate dichlorocarbene as an intermediate. | | Hazard | Toxic by ingestion, irritant to skin and eyes.
| | Flammability and Explosibility | Nonflammable | | Safety Profile | Moderately toxic by ingestion andintravenous routes. Human mutation data reported. Largedoses cause central nervous system depression. Used as anherbicide. Bags of the salt can ignite spontaneously instorage. When heated to decomposition it emits very |
| SODIUM TRICHLOROACETATE Preparation Products And Raw materials |
|