Other grades of this product :
| Ferrous oxalate dihydrate Basic information |
| Ferrous oxalate dihydrate Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 21/22 | | Safety Statements | 24/25 | | RIDADR | 3288 | | WGK Germany | 1 | | TSCA | Yes | | HazardClass | 6.1 | | PackingGroup | III |
| Ferrous oxalate dihydrate Usage And Synthesis |
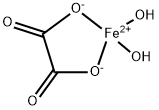
| Description | Iron(II) oxalate, FeC204.2H20, is precipitated as yellow crystals from solutions
containing iron(II) and oxalate ions ; in the presence of excess alkali metal oxalate, however,
soluble oxalato complexes M2[Fe(C2O4)2] are formed which can be precipitated by the
addition of alcohol. The oxalate is paramagnetic with μeff= 5·2 B.M. at room temperature. | | Chemical Properties | yellow powder
Ferrous oxalate, or iron(II) oxalate, is a chemical compound consisting of one iron(II) ion (Fe2) and one oxalate ion (C2O4(2−)). It has the chemical formula FeC2O4. Iron(II) oxalate is more commonly encountered as the dihydrate, FeC2O4·2H2O, CAS # 6047-25-2. Its crystal structure consists of chains of oxalate-bridged iron atoms, capped by water molecules. When heated, it dehydrates and decomposes into carbon dioxide, carbon monoxide, iron oxides and pyrophoric black iron.
| | Uses | It is used as photographic developer for silver bromide-gelatin plates. It imparts greenish-brown tint to optical glass (sunglasses, windshields, railroad car windows), for decorative glassware, pigment for plastics, paints and lacquers. It acts as reagent. | | Uses | It is used as photographic developer for silver bromide-gelatin plates. It imparts greenish-brown tint to optical glass (sunglasses, windshields, railroad car windows), for decorative glassware, pigment for plastics, paints and lacquers. It acts as reagent. |
| Ferrous oxalate dihydrate Preparation Products And Raw materials |
|