Other grades of this product :
| 3-(AMINOMETHYL)-PROXYL Basic information |
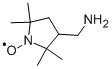
| Product Name: | 3-(AMINOMETHYL)-PROXYL | | Synonyms: | 3-aminomethyl-2,2,5,5-tetramethyl-1-pyrrolidinyl-n-oxyl;3-(AMINOMETHYL)-PROXYL;3-AMINOMETHYL-2,2,5,5-TETRAMETHYL-1-PYRROLIDINYLOXY;RARECHEM AL BW 2151;(3-Aminomethyl-2,2,5,5-tetramethyl-1-pyrrolidinyloxy)radical;3-Aminomethyl-2,2,5,5-tetramethylpyrrolidine-N-oxy;3-Aminomethyl-2,2,5,5-tetramethylpyrrolidine-N-oxyl;[3-(Aminomethyl)-2,2,5,5-tetramethyl-1-pyrrolidinyloxy]radical | | CAS: | 54606-49-4 | | MF: | C9H19N2O* | | MW: | 171.26 | | EINECS: | | Product Categories: | Heterocyclic Compounds;Heterocycles;Nitric Oxide Reagents | | Mol File: | 54606-49-4.mol |
| 3-(AMINOMETHYL)-PROXYL Chemical Properties |
| Boiling point | 128-131 °C13 mm Hg(lit.) | | Fp | 144 °F | | storage temp. | 2-8°C | | solubility | Chloroform, Dichloromethane, Dimethyl Sulfoxide, Ethyl Acetate | | form | Oil | | color | Yellow-Orange |
| 3-(AMINOMETHYL)-PROXYL Usage And Synthesis |
| Chemical Properties | Yellow-Orange Oil | | Uses | Useful spin label for studying biological systems. Used to study molecular diffusion into horse spleen ferritin and has been shown to be an effective nonthiol radioprotector.Used in pharmacological studies of myeloperoxidase-mediated hypochlorous acid production inhibitionReactant involved in: - Reduction reactions with nitroxides
- Synthesis of adenosine methycarboxamides for use as cardioprotectants
|
| 3-(AMINOMETHYL)-PROXYL Preparation Products And Raw materials |
|